(source)
(source)
|
Sir William Ramsay
(2 Oct 1852 - 23 Jul 1916)
Scottish chemist who was awarded the 1904 Nobel Prize in Chemistry for his “discovery of the inert gaseous elements in air.”
|
Radium and its Products
by Sir William Ramsay, K.C.B., F.R.S.
from Harper’s Magazine (Dec 1904)
[p.52] Chemistry and physics are experimental sciences; and those who are engaged in attempting to enlarge the boundaries of science by experiment are generally unwilling to publish speculations; for they have learned, by long experience, that it is unsafe to anticipate events. It is true, they must make certain theories and hypotheses. They must form some kind of mental picture of the relations between the phenomena which they are trying to investigate, else their experiments would be made at random, and without connection. Progress is made by trial and failure; the failures are generally a hundred times more numerous than the successes; yet they are usually left unchronicled. The reason is that the investigator feels that even though he has failed in achieving an expected result, some other more fortunate experimenter may succeed, and it is unwise to discourage his attempts.
In framing his suppositions, the investigator has a choice of five kinds; they have been classified by Dr. Johnstone Stoney. “A theory is a supposition which we hope to be true, a hypothesis is a supposition which we expect to be useful; fictions belong to the realm of art; if made to intrude elsewhere, they become either make-believes or mistakes.” Now the “man in the street,” when he thinks of science at all, hopes for a theory; whereas, the investigator is generally contented with a hypothesis, and it is only after forming and rejecting numerous hypotheses that he ventures to construct a theory. He has a rooted horror of fiction in the wrong place, and he dreads lest his hypothesis should turn out to be misplaced fiction.
I have thought it better to begin by these somewhat abstruse remarks, in order to place what I propose to discuss on a true basis. It is to be understood that any suppositions which I shall make use of are of the nature of hypotheses, devised solely because they may prove useful. Events are not yet ripe for a theory.
It will be remembered by the readers of this Magazine that Professor Rutherford and Mr. Soddy announced a “view ” that certain elements which possess the power of discharging an electroscope and which are therefore called “ radioactive,” are suffering disintegration—that is, they are splitting up into other elements, only one of which has as yet been identified. Three of these elements, namely, radium, thorium, and actinium, begin the process of disintegration by giving off an “emanation,” or supposed gas; the proof of the gaseous nature of these emanations is that they can be confined by glass or metal, like gases, and that they can be liquefied or solidified when cooled to a sufficiently low temperature. It is necessary to pay attention to this peculiarity; for these radioactive elements, and two others, uranium and polonium, also give off so-called β-rays, which penetrate glass and metal, and which are believed from the discoveries of Professor J. J. Thomson and others to be identical with negative electricity.
Now, Rutherford and Soddy, reasoning on the premises that radium was always found associated with uranium and thorium, and also that the ores of these metals, pitchblende and thorite, had been found to contain the gas helium, made the bold suggestion, “The speculation naturally arises whether the presence of helium in minerals and its invariable association with thorium and uranium may not be connected with their radioactivity.” Besides the premises already mentioned, they had evidence of the probable mass of the “ α-particles,” which appeared to be about twice that of an atom of hydrogen. Now. helium is the lightest gas next to hydrogen; and its atoms are four times as heavy as atoms of hydrogen. It was, therefore, a striking confirmation of the accuracy of this [p.53] view when Ramsay and Soddy discovered that helium can actually be obtained from radium.
Before giving an account of that discovery, a short description of the nature and properties of helium may not be out of place. When light passes through a prism, it is refracted, or bent; and Newton discovered that white light, such as is emitted from the sun or the stars, after passing through a prism, gives a spectrum consisting of colored images of the hole in the window-shutter through which the sunlight fell on his prism. Fraunhofer, a Berlin optician, conceived the idea of causing the light to pass through a narrow slit, instead of a round hole; and the spectrum then consisted of a number of images of the narrow slit, instead of the round hole. He was struck by one peculiarity shown by sunlight, when thus examined, namely, that the colored band, rainbow-like, and exhibiting a regular gradation of color from red at the one end, through orange, yellow, green, and blue, to violet at the other end, was interspersed by very numerous thin black lines. The nature of these lines was discovered by Kirchhof. The light emitted by a white-hot body shows a continuous spectrum; but if such white light be passed through the vapors of a metal, such as sodium, a portion is absorbed. For example, glowing sodium gas shows two yellow lines, very close together; but if this light is passed through the vapor of sodium, these lines are extinguished if the correct amount of vapor be interposed. Now it was found that the position of the two dark lines in the sun's spectrum, discovered by Fraunhofer, is identical with that of the two yellow lines visible in the spectrum of glowing sodium vapor; and Kirchhof concluded that this coincidence furnished a proof of the presence of sodium in the sun. Fraunhofer had named these lines D1 and D2, Similar conclusions were drawn from observations of the coincidence of other black solar lines with those of elements found on the earth; and the presence of iron, lead, copper, and a host of elements in the sun was proved.
In 1868 a. total eclipse of the sun took place; an expedition was sent to India, from which a good view was to be obtained. Monsieur Janssen, the distinguished French astronomer, observed a yellow line, not a dark, but a bright one, in the light which reached the earth from the edge or “limb” of the sun, and which proceeded from its colored atmosphere or chromosphere. It was for some time suspected that this line, which was almost identical in position with the yellow lines of sodium, D1 and D2, and which Janssen named D3 was due to hydrogen. But ordinary hydrogen had never been found to show such a line; and after Sir Edward Frankland and Sir Norman Lockyer had convinced themselves by numerous experiments that D3 had nothing to do with hydrogen, they ascribed it to a new element, the existence of which on the sun they regarded as probable; and for convenience, they named this undiscovered element “helium” from the Greek word for the sun, ἥλιος.
It was not until the year 1895 that helium was found on the earth. After the discovery of argon in 1894, Ramsay repeated some experiments which had previously been made by Dr. Hillebrand, of the United States Geological Survey. Hillebrand had found that certain minerals, especially those containing the somewhat rare elements uranium and thorium, when heated, or when treated with acids, gave off a gas which he took for nitrogen. But the discovery of argon had taught Ramsay how to deal with such a gas. He examined it in the hope that it might lead to the discovery of a compound of argon; but its spectrum turned out to be identical with that of solar helium. and terrestrial helium was discovered. It proved to be a very light gas, only twice as heavy as hydrogen, the lightest substance known; its spectrum consists of nine very brilliant lines, of which D3 is the most brilliant; it has never been condensed to the liquid state, and is the only gas of which that can now be said (for hydrogen has been liquefied within the last few years), and, like argon, it has not been induced to form any chemical compound. That it is an element is shown by the relation of its atomic weight, 4, to that of other elements, as well as by certain of its properties, the most important of which is the ratio between its specific heat at [p.54] constant volume and constant pressure; but to explain the bearing of this property on the reasoning which proves it to be an element would be foreign to the subject of this article.
This, then, was the elementary substance that Rutherford and Soddy suspected to be one of the decomposition products of radium. The word “decomposition,” however, implies the disruption of a compound, and the change which takes place when radium produces helium is of such a striking nature that it is perhaps preferable to use the term “disintegration.”
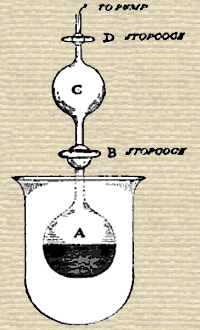
Having procured fifty milligrammes (about three-quarters of a grain) of radium bromide, Ramsay and Soddy placed the grayish-brown crystalline powder in a small glass bulb about an inch in diameter. This bulb was connected by means of a capillary tube with another bulb of about the same size; on each side of the second bulb there was a stop-cock, as shown in the sketch. To begin with, the bulb A was pumped empty of air; it contained the dry bromide of radium. The stopcock B was then shut. Next, some water was placed in bulb C, and it, too, was pumped free from air, and the stop-cock D was closed. B was then opened, so that the water in C flowed into A, and dissolved up the bromide of radium. As it was dissolving, gas bubbles were evolved with effervescence, and that gas collected in the two bulbs, A and B. The sketch shows the state of matters after the water had been added and the gas evolved. The apparatus was then permanently sealed on to a tube connected with a mercury-pump, so contrived that gas could be collected. The stop-cocks having opened, the gas passed into the pump, and was received in a small test-tube. From the test-tube it was passed into a reservoir, where it was mixed with pure oxygen, and electric sparks were then passed through it for some hours, a little caustic soda being present. This process has the result of causing all gases except those like argon to combine, and they are therefore removed. It was easy to withdraw oxygen by heating a little phosphorus in the gas; and it was then passed into a small narrow glass tube, which had a platinum wire sealed in at each end—a so-called Plücker’s vacuum-tube. On passing an electric discharge from a Ruhmkorff induction-coil through the gas in the tube, the well-known spectrum of helium was seen.
Thus helium was proved to be contained in radium bromide which had stood for some time. The specimen used was said to be about three months old, and the helium had accumulated. But whence came the helium? That was the next question to be settled.
A solution of radium bromide gives off gas continuously. That gas, on investigation, is found to be a mixture of oxygen and hydrogen, the constituents of the water in which the bromide is dissolved. It contains, however, a small excess of hydrogen, which implies that some oxygen has been absorbed, probably by the radium bromide, although what becomes of that excess has not yet been determined.
When an electric spark is passed through a mixture of oxygen and hydrogen, an explosion takes place; the gases combine, and water is formed. Any excess of hydrogen is, however, unaffected. Now, the gases evolved from a solution of radium bromide are luminous in the dark, and possess the power of discharging an electroscope, like radium bromide itself. Rutherford and Soddy discovered that when this mixture of gases is led through a tube shaped like a U, cooled to –185° C. by dipping in liquid air, the luminous gas condenses, and the gases which pass on have nearly ceased to be luminous in the dark, and no longer discharge an electroscope. To such condensable gases Rutherford applied the term “emanation ”; this one is known as the “radium emanation.”
[p.55] The next question to be answered was: Is the helium evolved from the radium bromide directly, or is it a product of the emanation? It was necessary, therefore, to collect the emanation and to examine its spectrum. This was managed, after many unsuccessful trials, by exploding the mixture of oxygen and hydrogen containing the emanation, allowing the remaining hydrogen to pass into a tube containing a thin spiral of slightly oxidized copper wire kept at a red heat by an electric current: the hydrogen combined with the oxygen of the oxide of copper, and formed water. The apparatus was so arranged that mercury could be allowed to enter the tube from below, so as to sweep before it any remaining gas; and the water was removed from the gas by making it pass through a tube filled with a suitable absorbing agent, followed up by mercury. The gas finally entered a very small spectrum-tube, entirely made of capillary tubing, like the stem of a thermometer. On passing a discharge from a coil through the spectrum-tube after the emanation had been thus introduced, a spectrum was seen, consisting of some bright green lines; but it was extremely difficult to prevent the presence of traces of carbon compounds, and at this stage their spectrum was always seen. But the D3 line of helium was absent. After a couple of days, however, a faint yellow hue began to appear, identical in position with D3; and as time went on, that line became more distinct, and was followed by the other lines characteristic of helium, until, after a week, the whole helium spectrum was visible. It was thus proved that the radium emanation spontaneously changes into helium. Of course other substances might have been, and undoubtedly were, formed; but these it was not possible to detect.
The next problem was to measure the amount of emanation, resulting from a given weight of radium, in a given time. The method of procedure was similar to that already described, except in one respect: the spiral of oxidized copper wire was omitted, and the excess of hydrogen, mixed with the emanation, was cooled in a small bulb by help of liquid air. This condensed the emanation; and the hydrogen, which of course is not liquefied at the temperature of liquid air, was pumped away. On removal of the liquid air the emanation became gaseous, and it was forced by means of mercury into a minute measuring tube, like the very narrow stem of a thermometer. It was thus possible to measure its volume. It is a well-known law that gases decrease in volume proportionally to increase of pressure; if the pressure is doubled, the volume of the gas is halved, and so on. Now this was found to be the case with the emanation; hence the conclusion that it is a gas, in the ordinary meaning of the word. But it is a very unusual gas; for not only is it luminous in the dark, but it slowly contracts, day by day, until it practically all disappears. It does not lose its luminosity, however; what remains, day by day, is as luminous as ever; but its volume decreased, until after about twenty-five days the gas had contracted to a mere luminous point. What had become of the helium? That was discovered on heating the tube. It is well known that glass, exposed to the radium emanation, turns purple, if it is soda glass; brown, if it is potash glass. This is due to the penetration of the glass by the electrons, which are exceedingly minute particles, moving with enormous velocity. When the emanation changes into helium, the molecules of that gas are also shot off with enormous velocity, although they move much more slowly than the electrons. It is sufficient, however, to cause them to penetrate the glass; but on heating they are evolved, and collect in the tube, and the volume of the helium can be measured. It turned out to be three and a half times that of the emanation. But as the emanation is probably fifty times as heavy as hydrogen, all the emanation is not accounted for by the volume of helium found; it is almost certain that solid products are formed, which are deposited on the glass, and which are radioactive. Up to the present these products have not been investigated.
It was possible, knowing the volume of the emanation, and knowing also the volume which the radium would have occupied had it, too, been gaseous (for a simple rule enables chemists to know the volume which a given weight of any element would occupy in the state of gas). to calculate how long it would take [p.56] for the radium to be converted into emanation, supposing that to be its only product. This gives for half of the radium to be decomposed about 1150 years. But there is a good deal of conjecture about the calculation; for many unproved assumptions have to be made.
A further experiment, conducted in a somewhat similar manner, but with the utmost precaution to exclude every trace of foreign gas, made it possible to measure the position of the lines of the spectrum of the emanation. In general it may be said that the spectrum has a similar character to those of argon and helium; it consists of a number of bright lines, chiefly green, appearing distinctly on a black background. It confirms the supposition, made after examination of the chemical properties of the emanation, that it is a gas belonging to the argon group, with a very heavy atomic weight. Some of the lines of the spectrum appear to be identical with lines observed in the spectra of the stars; and it may perhaps be inferred that such heavenly bodies are rich in radium.
If the diagram on page 54 be looked at, it will be seen that the bulb containing radium bromide was surrounded by a small beaker, as a precautionary measure. As a matter of fact, there were three such bulbs and three such beakers, on the principle of not putting all one’s eggs in one basket. These beakers had never been in contact with the radium bromide, nor with the emanation; but they had been bombarded for months by β-rays, or electrons, which are so minute, and move so rapidly, that they penetrate thin glass with ease. It was found that these beakers were radioactive; and it is very remarkable that after washing with water, the beakers lost their radioactivity, which was transferred to the water. Evidently, then, some radioactive matter had been produced by the influence of the β-rays. On investigation, it was proved that more than one substance had been produced. For on bubbling air through the water, a radioactive gas passed away along with the air; it had the power of discharging an electroscope, but its life lasted only a few seconds. It was only while the current of air was passing through the electroscope that the gold-leaves fell together; on ceasing current, the leaves remained practically stationary. Now had radium emanation been introduced into the electroscope, its effect would have lasted twenty-eight days; had the emanation from thorium been introduced, it would have taken about a minute before it ceased to cause the gold-leaves to fall in. There is an emanation, however, that from actinium, which is very short-lived, and it looks probable that one of the substances produced from the β-rays is actinium. But it is not the only one. For the water with which the glass was washed gives a radioactive residue after evaporation to dryness; and it contains a substance which forms an insoluble chloride, sulphide, and sulphate, though the hydroxide is soluble in ammonia. Either, then, the β-rays have so altered the constituents of the glass that new radioactive elements are formed; or perhaps it is the air which surrounds the glass which has yielded these new elements; or it may be, though this appears less probable, that the β-rays themselves, which are identical with electrons, or “atoms” of negative electricity, have condensed to form matter.
Such are some of the results which have been obtained in a. chemical examination of the products of change of radium. The work is merely begun, but it leads to a hypothesis as regards the constitution of radium and similar elements, which was first put forward by Rutherford and Soddy. It is that atoms of elements of high atomic weight, such as radium, uranium, thorium, and the suspected elements polonium and actinium, are unstable; that they undergo spontaneous change into other forms of matter, themselves radioactive, and themselves unstable; and that finally elements are produced which, on account of their non-radioactivity, are, as a rule, impossible to recognize, for their minute amount precludes the application of any ordinary test with success. The recognition of helium, however, which is comparatively easy of detection, lends great support to this hypothesis.
The natural question which suggests itself is: Are other elements undergoing similar change? Can it be that their rate of change is so slow that it cannot be detected? Professor J. J. Thomson [p.57] has attempted to answer this question, and he has found that many ordinary elements are faintly radioactive; but the answer is still incomplete, for, first, radium is so enormously radioactive that the merest trace of one of its salts in the salt of another element would produce such radioactivity; and, second, it is not proved that radioactivity is an invariable accompaniment of such change; or again, it may be evolved so slowly as to escape detection. A lump of coal, for example, is slowly being oxidized by the oxygen of the air; oxidation is attended by a rise of temperature, but the most delicate thermometer would detect no difference between the temperature of a lump of coal and that of the surrounding air, for the rate of oxidation is so slow.
Another question which arises is: Seeing that an element like radium is changing into other substances, and that its life is a comparatively short one, it must be in course of formation, else its amount would be exhausted in about 2500 years. An attempt has been made by Soddy to see if uranium salts, carefully purified from radium, have reproduced radium after an interval of a year; but his result was a negative one. Possibly some other form of matter besides uranium contributes to the synthesis of radium, and further experiments in this direction will be eagerly welcomed.
Lastly, the experiments of Ramsay and Cook, of which an account has been given in the foregoing page, on the action of the β-rays appear to foreshadow results of importance. For while radium, during its spontaneous change, parts with a relatively enormous amount of energy, largely in the form of heat, it is a legitimate inference that if the atoms of ordinary elements could be made to absorb energy, they would undergo change of a constructive, and not of a disruptive, nature. If, as looks probable, the action of β-rays, themselves the conveyers of enormous energy, on such matter as glass, is to build up atoms which are radioactive, and consequently of high atomic weight; and if it be found that the particular matter produced depends on the element on which the β-rays fall, and to which they impart their energy:– if these hypotheses are just, then the transmutation of elements no longer appears an idle dream. The philosopher’s stone will have been discovered, and it is not beyond the bounds of possibility that it may lead to that other goal of the philosophers of the dark ages—the elixir vitæ. For the action of living cells is also dependent on the nature and direction of the energy which they contain; and who can say that it will be impossible to control their action, when the means of imparting and controlling energy shall have been investigated?
- Science Quotes by Sir William Ramsay.
- 2 Oct - short biography, births, deaths and events on date of Ramsay's birth.
- How Discoveries Are Made - by William Ramsay in Cassell’s Magazine (1908)
- The Early Days of Chemistry - by William Ramsay, Collected in Essays Biographical and Chemical (1909).
- Air: Scientific Discoveries and Inventions - from Haydn's Dictionary of Dates (1904).
- A Life of Sir William Ramsay, by Morris W. Travers. - book suggestion.