107Stories About ChemistryINDEX |
59.
The Reason for and the Consequences of Diversity
Carbon atoms form chains very readily, arranging themselves one after the other in long lines. The shortest chain has two carbon atoms. For example, the molecule of the hydrocarbon ethane has two links in its chain: H3C�CH3. Well, and what about the longest? This is unknown so far. Compounds with 70 carbon links in their chain have been obtained (It should be noted that ordinary compounds are meant here, and not polymers. In the latter case the hydrocarbon chains may be much longer.)  None of the other elements can do anything of the kind. Only silicon allows itself the luxury of forming a six-link chain. And scientists have obtained a curious compound of germanium, the hydrogen germanide Ge3H8, in which three metallic atoms are linked into a chain This is the only case of its kind among the metals To put it briefly, carbon has no rival in �chain-formation capacity.� But if carbon chains were only linear, organic chemistry would never know such a fabulous number of compounds. Chains are capable of branching and of closing up into cycles. These are polygons, consisting of three, four, five, six, and more carbon atoms The chain of the hydrocarbon butane consists of four carbon atoms. 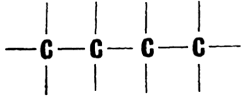 Here the atoms are �lined up�. But they may take up the following positions: 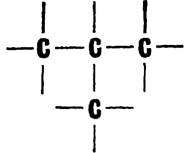 The number of atoms is the same, but their arrangement is different. And the second formula represents a different substance with other properties and a different name, isobutane. Five carbon atoms can form five branched chains besides the linear one. And each of these structures represents a separate chemical substance. Chemists have thought up a special name for varieties of chemical compounds which contain the same atoms arranged differently: they are called isomers The more carbon atoms in the molecule, the larger the number of isomers. As a matter of fact, their number grows almost in geometrical progression. And this adds hundreds of thousands of new compounds to the reserves of organic chemistry |





