107Stories About ChemistryINDEX |
66.
A Eulogy to Cadet�s Liquid
In 1760 the little known French chemist Cadet made history without suspecting it. In his laboratory he performed the following chemical experiment (we have no idea what for). Cadet heated potassium acetate with arsenic oxide. What the result was he never found out, because the substance that formed was of a truly diabolical disposition. It was a black, thick liquid. It fumed in the air, and burst into flame readily. Besides, it had an utterly unbearable odour.  Cadet�s �concoction� was analysed about seventy years later. Its main components were found to be arsenious compounds of a very singular nature. To understand what was so singular about them it must be recalled that all organic compounds have a very important feature in common: they are based on chains of carbon atoms, straight, branched, or cyclic. True, atoms of some other elements may wedge into these chains. But there are very few such elements (they are called organogens): oxygen, nitrogen, hydrogen, sulphur, and perhaps, phosphorus. Arsenic is decidedly not one of them. Cadet�s liquid contained a substance called cacodyl (from the Greek �kakodes� meaning ill-smelling). Its constitution is such that the arsenic atoms have wormed themselves firmly in between the carbon atoms, thus: 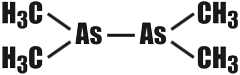 Organic compounds whose carbon chains include atoms of inorganogenic elements (metals or nonmetals) are now called hetero-organic compounds (organometallic, if the element is a metal). Thus, Cadet synthesized the first hetero-organic compound in the world. Nowadays more than 15 thousand such substances are known. Hetero-organic, and especially organometallic chemistry has become a large independent branch of chemistry, one of its more important chapters. It has bridged the gap between organic and inorganic chemistry and has shown once again how conventional the subdivision of the sciences is in our times. Indeed, what kind of organic chemistry is it that deals with compounds in which metals, typical representatives of inanimate nature, play the most important part? And, contrariwise, can it be called inorganic chemistry if a large number of its subject substances are in many respects purely organic derivatives? Of major interest to science are organometallic compounds. Their compulsory attribute is the bond between a metallic atom and a carbon atom. Almost all the metals of the main subgroups of the Big House may be contained in organometallic compounds. The properties of these substances are very diverse. Some explode with a terrible force even at temperatures far below zero. Others, on the other hand, possess great resistance to heat. Some are chemically very active, while others respond reluctantly to all kinds of external influences. And every last one of them is poisonous, except for the organometallic compounds of germanium. Why the latter are harmless is still a riddle. The range of applications of hetero-organic compounds is very broad, and practically inexhaustible. They are used in the preparation of plastics and rubbers, in the manufacture of semiconductors and superpure metals; as medicinals and agricultural pesticides and as components of rocket and motor fuels; finally, they are very valuable chemical reagents and catalysts, enabling the successful accomplishment of many important processes. Our country has a large school of hetero-organic chemists, headed by Academician Alexander Nesmeyanov who was recently awarded the Lenin Prize for his work. |





