107Stories About ChemistryINDEX |
93.
Endless Molecules
Everyone knows what rubber is. It is balls and galoshes. It is the hockey puck and the surgeon�s gloves. It is automobile tyres and hot-water bottles, waterproof raincoats and hoses. Rubber and rubber goods are put out nowadays by hundreds of factories and plants. A few decades ago all rubber goods were made of natural rubber, otherwise called caoutchouc. The word �caoutchouc� comes from the Tupi* �caou-uchu� meaning �tears of the Hevea.� [* Tupi is the language spoken by a group of South American Indian tribes inhabiting mainly the valley of the Amazon.�Tr.] The Hevea is one of the most important trees whose sap is used to make rubber. Many useful things can be made from rubber, and it is too bad that its production is very labour-consuming, and that the Hevea grows only in the tropics. All this has made it impossible to meet the demands of industry for the natural raw material. Here again chemistry came to the rescue First of all, chemists tried to find the answer to the question of why rubber is so elastic After studying the �tears of the Hevea� for a long time they finally found the answer. It proved that the rubber molecules have a very peculiar structure. They consist of a large number of recurring identical units linked into immense chains. Of course, a long molecule consisting of about fifteen thousand units, can bend in all directions and is elastic. It was found that the units making up the chain are molecules of the hydrocarbon isoprene, C5H8, which has the following structural formula: 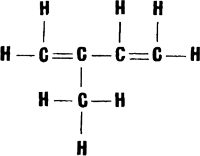 It would be more correct to say that isoprene is a sort of initial natural monomer. In the course of polymerization the isoprene molecule undergoes a slight change: the double bonds between the carbon atoms open, and the freed bonds link up the units into the gigantic rubber molecules. The problem of producing synthetic rubber drew the attention of scientists and engineers long ago. At first glance the job did not seem very tricky One had to produce isoprene and then make it polymerize, i.e., make the isoprene units combine into long and flexible chains of artificial rubber. But reality was disappointing. Chemists succeeded with some difficulty in synthesizing isoprene, but when it came to polymerizing it rubber did not result. The units combined with each other, but did so haphazardly rather than in a definite order and the result was artificial products which resembled rubber slightly but differed from it in many respects. And so the chemists had to invent methods of making the isoprene units connect up into a chain in the right way The world�s first industrial synthetic rubber was produced in the Soviet Union. Academician S. Lebedev chose a different substance, butadiene, for the chain unit: 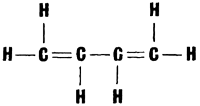 It greatly resembles isoprene in composition and structure, but its polymerization is easier to control. At present quite a large number of synthetic rubbers are known (they are now often called elastomers to distinguish them from natural rubber). Natural rubber and goods made from it have substantial shortcomings. For example, it swells greatly in oils and fats, displays little resistance to many oxidants, particularly ozone, traces of which are always present in the atmosphere. Articles made of natural rubber have to be vulcanized, i.e., treated with sulphur at a high temperature That is how raw rubber is converted into cured rubber or ebonite. In service, goods made of natural rubber (e.g., automobile tyres) evolve a large amount of heat which causes aging and rapid wear.  That is why scientists had to create new synthetic rubbers possessing better properties. For instance, there is a family of rubbers known as �Buna� The name is derived from the initial letters of two words �butadiene� and �natrium� (the Latin for �sodium�), sodium playing the part of the catalyst during polymerization Some of the elastomers of this family possess excellent properties and are used mainly for the production of vehicle tyres. Of especially great importance is butyl rubber, produced by the joint polymerization of isobutene and isoprene. First of all, it is the cheapest rubber Secondly, in contrast to natural rubber it is almost indifferent to ozone Besides, butyl rubber vulcanizates used extensively at present for the manufacture of tyre tubes are ten times as impervious to air as vulcanizates made of natural rubber. Of special interest are polyurethane rubbers. They possess high tensile strength and are almost unaging. Polyurethane elastomers are used to make foam rubber for upholstery. Rubbers have been developed in recent years which scientists could not even dream of before. These are primarily elastomers based on organosilicon and fluorocarbon compounds The thermal stability of these elastomers is twice as high as that of natural rubber. They are resistant to ozone, and rubber based on fluorocarbon compounds resists even fuming sulphuric and nitric acids. But this is not all. A recent addition are the carboxyl-containing rubbers, copolymers of butadiene and organic acids They have a very high tensile strength. It is thus evident that in this field too nature has yielded its superiority to man-made materials. |





