|
This experiment was repeated by Sir James Chadwick who showed the effect was due to a new type of . particle which had the mass of a hydrogen nucleus but carried no electric charge. He gave it the name "Neutron." The existence of the Neutron had been forecast by Rutherford 12 years before. Three years later, in 1935, Professor Arthur Dempster of the University of Chicago, using a mass spectrometer detected a rare atom of uranium with atomic weight of 235. The more common uranium has a weight of 238 on the scale where oxygen is 16. 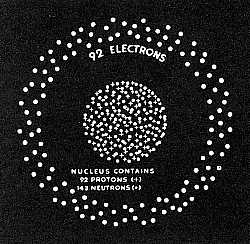 "U235" All radioactive materials disintegrate and in so doing give off energy with the loss of weight. Ordinary disintegration, such as that of radium, is a slow continuous process. In 1939, Hahn, Strassman, Meitner and Frisch discovered a new type of atomic disintegration which is a violent process and is started by bombarding the nucleus of the atom with slow moving neutrons. The most familiar substance which exhibits this type of fragmentation is U235. Once the process starts it accelerates and releases a tremendous amount of energy. |








