MINERAL WOOL.
from Iron Age, as reprinted in Scientific American (12 Jan 1878)
A small portion of the waste product of a blast furnace may be successfully utilized by converting it, while in the molten state, into the material known as mineral wool. The cheapness of the slag and the simplicity of the process render the manufacture of mineral wool capable of being carried on in all iron districts, and it is the object of this article to give such an explanation of its properties and characteristics as may be of general interest.
The process is confined to the treatment of substances containing a high percentage of silica. Regarding slag as a silicate of lime and magnesia and alumina, we can readily see that, as the silica acts as the acid, its presence in a low or high degree gives wide range for difference in the resulting slags: and according to the predominance of either base or acid, they are classed as basic, acid or neutral. A great variety of mineral wools is thus afforded, but experience has shown that a neutral slag produces finer fibers than one containing a large amount of silica, and therefore it is more pliable and easier of application. Slags containing manganese make a greenish wool, while pink or reddish wool appears to accompany hard grades of iron. Under other circumstances the wool will be white, being purest white when the percentage of base is large and assuming a gray and smoky color as the silica increases.
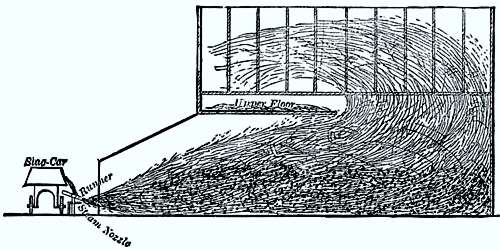
The diagram shows a section of the mineral wool house and apparatus at the Clove Furnace, Greenwood Iron Works, Orange County, N. Y. At this furnace, as at the majority of American furnaces, there is not sufficient room about the stack to erect a chamber in which to blow the mineral wool, and even if there was ample space it would be economy to allow the slag first to run into box-cars and afterward tap these, because the cinder, when it comes directly from the furnace, flows too fast to be controlled and properly utilized. As a result of this difficulty the wool-house stands about 100 feet from the furnace. When a car is run full of fluid cinder it is taken in front of the wool-house, the chilled slag knocked out of the tap-hole, and a small stream only allowed to fall to the runner, over which it travels and again falls 3½ inches, where it is met by the jet of steam.
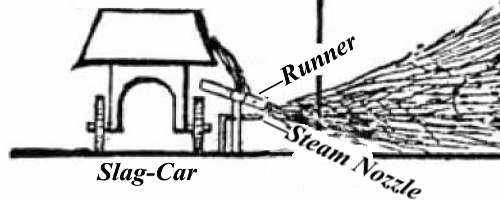
The sudden impact of steam or air under pressure against thinly flowing slag immediately scatters the stream, forming a spray of small globules or shot, which, on becoming detached from the larger mass, stick, as it were, and thus give a beginning to the vitreous thread.
The aperture of the nozzle which forms the jet of steam plays a most important part in separating the stream of slag into small shot at the outset, for it is obvious that the smaller they are at the first the greater the possibility of their being absorbed in the fiber, which is pulled out in their flight. The blowing part of the process has been so far improved that the shot are much less than a sixteenth of an inch in diameter, and there is no need of separating them from the wool, as they do not impair its effect.
The wool house is 30 feet long by 14 wide; the gable of the roof is 21 feet above the floor, and a sheet iron extension 10 feet long reaches to the slag car. The frame of the building is covered on the interior with thin sheet iron. The front of the house is provided with a window which serves as an outlet for the currents created by the jet of steam, and these currents carry a portion of the lightest wool up on the top floor. The mineral wool taken from the first floor is entirely free from shot, and is called No. 3; the other two grades are deposited on the lower floor, and are separated also by currents of air.
As this conversion of vitreous substances into a filamentous or fibrous material is a mechanical one, we find the wool to be of the same composition as the slag, so that, if the composition of a slag be previously ascertained, the character of the resulting wool may, in a measure, be determined.
The density of bodies, generally speaking, determines the rapidity with which heat is transmitted through them, and accordingly we find the metals to be best conductors, and stone-earthenware and plaster not so good, while porous or air-confining substances constitute the so-called non-conductors. The gases are very poor conductors, and probably air is the worst conductor known, that is, it is the substance which, when at rest, impedes the passage of heat most. But heat is conveyed through air by the movement of its particles, which is obviously not the case in more dense media; therefore, we should infer that, if the circulation of the air were some suitable absorbent, the passage of heat would be retarded. This brings us to a clear conception at last of what a non-conductor ought to be, and it might not be out of place here to recall to mind the fact that birds are clothed with feathers and beasts with hair as a protection against cold, and that these coverings are poor conductors, simply because they hold in suspension an enormous amount of air. In order to ascertain how much air is confined in mineral wool, we will consult figures a little. A cubic foot of slag weighs 192 lbs., while a cubic foot of No. 1 mineral wool weighs but 28 lbs.; No. 2, 16 lbs. and No. 3, 8 lbs.; thus showing as a result of the conversion a decrease in weight, which is equivalent to an increase in air-space of 85 per cent., 90 per cent., 95 per cent., for the three grades respectively. The immense expansion is better illustrated, perhaps, by saying that one cubic foot of slag will make 24 cubic feet of No. 8 mineral wool, which would cover 192 square feet two inches thick.
A substance, to be a superior non-conductor for application on heated surfaces, should combine great air-confining capacity with indestructibility, which means that it should contain nothing organic. To further substantiate these statements, which might otherwise appear hypothetical, a few experiments have been made after the method used by Count Rumford in 1792, when he ascertained the relative degrees in which furs, feathers and other organic materials used for clothing conduct heat.
The ball and stem of a thermometer were covered with a inch thickness of the substance to be tried, by placing it within a larger bulb of glass and then filling the surrounding interval between the two with the substance; and after heating this apparatus to a given degree in boiling water, it was surrounded by ice, and the comparative times required to cool the thermometer a certain number of degrees was noted. The figures following the names of the substances mark the number of records required respectively for cooling down the thermometer through 60 degrees Fahrenheit: Asbestos, 390; cotton, 438: felt, 463; mineral wool, 770. Of course cotton has no bearing on the subject under discussion, except that it is used for domestic purposes as a poor conductor of heat. but asbestos and felt are used extensively to protect heated surfaces.
It is a characteristic of organic substances that they gradually become impaired by heat, are liable to burn; and there is no reason to suppose that felt has lost this unfortunate property. There is a disparity in the weights of these substances which favors the felt. The space filled being the same in all cases, the relative weights were noted as follows Felt, 810 grains; mineral wool, 757 grains; asbestos, 2483.
For the coating of steam boilers, cylinders, steam domes, pipes, etc., mineral wool is especially valuable. It is economic, durable, and very easy of application. It can be applied underneath wooden lagging or sheet iron. These can be kept at a uniform distance from the boiler by runners or studs, and the mineral wool stuffed under as the lagging is put on. The strips forming the lagging should be tongued and grooved and seasoned to prevent their warping afterward. The wool must not be stamped in so as to crush it, but must simply be loosely pressed, so as to thoroughly fill the open spaces. For the up take in marine boilers, which are often carelessly covered with combustible materials, and cause frequent alarms of fire, mineral wool is particularly adapted, because it cannot possibly burn. Thin sheet iron makes a very neat and lasting jacket, and is readily bent over the boilers before the wool is stuffed in.
In consequence of the looseness of mineral wool, its application to pipes requires some device for holding it on. Where a pipe runs underground or in the open air, a common box answers every purpose, and for inside work canvas makes a suitable jacket, if it is properly kept in place by collars or studs. Vulcanized fiber is a material admirably adapted for a covering, because it is pliable and yet of sufficient stiffness to keep in shape while packing. It has a brown color, and is generally varnished so as to withstand the weather, it should be put on in sections of about a foot at a time, and secured by small brass clasps placed at intervals of 8 or 4 inches.
Mineral wool should be applied between 2 and 3 inches thick. The No. 1 quality is used for lining large ice houses, brewers’ vaults, etc., and is put in with best results 4 inches thick, at a cost of 12½ cents per square foot. A square foot of No. 2, two inches thick, costs 10 cents, and one of No. 8 costs 20 cents. This material is receiving wide introduction in England under the name of slag wool, and it has also been in very general use in Germany, where it is called silicate cotton. The process for the manufacture of mineral wool and its manipulation are protected by five United States patents, the rights for the use of which in the different States or parts of them must be secured from Mr. A. D. Elbers, 26½ Broadway, N.Y. Mr. Elbers is the sole agent for the sale of mineral wool in this country.—Iron Age.
- Mineral Wool or Mineral Cotton - article from Appleton's Annual Cyclopaedia for 1891
- Mineral Wool From Limestone - article from Stone Magazine (1898)
- What is this mineral wool? - cartoon from the New York Times (7 Mar 1897)
- Today in Science History event description for the opening of Crystal Chemical Works rock wool factory on 1 Jun 1897.




