(source)
(source)
|
Henri Becquerel
(15 Dec 1852 - 25 Aug 1908)
French physicist who discovered radioactivity in fluorescent salts of uranium, for which he shared the 1903 Nobel Prize for Physics with Pierre and Marie Curie.
|
Radio-Activity
A New Property of Matter
by Robert Kennedy Duncan
Professor of Chemistry, Washington and Jefferson College
From Harper's Magazine (1902)
[p.356] “In the beginning God created,” and in the midst of His creation He set down man with a little spark of the Godhead in him to make him strive to know,—and in the striving, to grow, and to progress to some great, worthy, unknown end in this world. He gave him hands to do, a will to drive, and seven senses to apprehend,—just a working equipment; and so he has won his way, so far, out of the horrible conditions of pre-history.
To know, is to work and to do; and a new thing done is forever a rung in the ladder by which man climbs,—necessary, and good for all generations, until the summit is attained and the ladder can be cast aside.
The theme of this present article is of a new Thing Done—the discovery of a new property of matter. All we can do is simply to place it on its feet before you in a collection of experiments. It is hoped that outside of its extrinsic interest you will see deep within it the beauty and the poetry of reasoned Action.
If you questioned the discoverer—the doer of the work—about himself, he would probably tell you that his work, possibly, was something—he himself was nothing; and in a measure he is right; for in a few years he will pass, while his work will endure forever. Still, we wish to know him for his work's sake, and surely it will not be amiss to say something at least about him.
Let us say, then, that Henri Becquerel, Membre de l'Institut, is the discoverer of Becquerel rays, the basis of the phenomena of radio-activity. He comes very honestly by his powers. His grandfather, Antoine-César (1788-1878), through sixty years of indefatigable labor, contributed more than five hundred memoirs, works of note on mineralogy and electricity. His father, Alexandre-Edmond (1820-1891) was the author of so many memoirs that they constitute practically a history of the relations of optics to electricity through the past fifty years. Henri Becquerel, the son, was subjected to the training and influence of these honored men, and it is little wonder, then, that, through heredity and environment, he should bear the face of one who sends his soul into the invisible—for that, in good solid truth, is what every true experimenter literally does. In due time he succeeded to the Professorship of Physics, the chair of his fathers, and began his work in their laboratory in the quaint old home of Cuvier in the Jardin des Plantes,—“a laboratory to which I had gone,” he says, “from the time I was able to walk.” There he wrought nobly for the credit of his name, until Rontgen's discovery of the X-rays initiated an investigation which culminated in the discovery of the Becquerel rays and radio-activity.
Now Becquerel did not discover his rays and their radio-activity out of nothing. Every scientific discovery has a genealogy of its own, going back to the primal ancestor of all thoughts; no discovery comes into the world parentless of previous conceptions. On the following page is a table showing a few steps in the genealogy of the Becquerel rays.
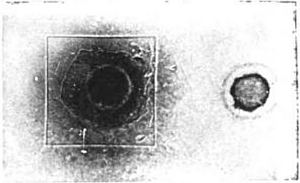
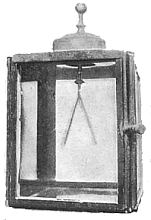
X-rays are in some way entangled with the phosphorescence in a Crookes tube. Consequently the discovery of Professor Röntgen set men wondering as to whether the power of emitting penetrating rays might not be a property of phosphorescent bodies in general. Thought always advances in waves; and [p.357] there are always several men on the top of the same wave. In this instance was Niewenglowski, who made the interesting discovery that some such rays were actually emitted: that much at least. His experiment, as afterward repeated by Becquerel, is perfectly demonstrative. A certain compound of sulphur and calcium, calcium sulphide, which is the basis of luminous paint, shines in the dark after exposure to sunlight— that is, it is phosphorescent. Niewenglowski placed a photographic plate in a plate-holder, and, instead of a coverslide, he inserted a thin sheet of aluminum. The plate was thus completely sheltered from the action of light. Upon the sheet of aluminum he placed squares of thin glass, and upon these, in turn, pieces of a certain calcium sulphide previously exposed to light, which were protected from external influences by dome-shaped clock-glasses. The arrangement of the experiment is seen in Fig. 1—the cover-slide of aluminum, the glass, the sulphide above them, and the clock-glasses covering all.
The apparatus was left in the dark for twenty-three hours. The plate was then developed. A print from the actual plate, which is here reproduced (Fig. 2) leaves no muddy obscurity for the reader. Upon the plate are imprinted the square of glass and the round section of the clockglass cover. The rays had, necessarily, to pass straight through the aluminum cover-slide to print them there. It was thus, then, that the question was asked of Nature, “Does this substance, this calcium sulphide, emit rays which will penetrate glass and metal to affect a photographic plate?” And Nature answered, there in her legible signature, “This substance will.”

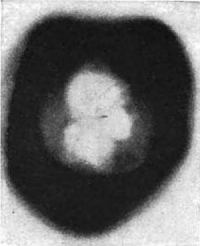
But men are children, and one question fathers another. Are these rays light. The answer is upon the same plate. It is affirmative. If you examine the imprint of the square of glass upon this plate, you will notice that it is bordered by a perfectly white line which has been left untouched by the rays. This can only be accounted for by supposing that they were bent, or refracted, on passing through the edges of the glass into the air.
Now rays that are made up of particles, such as cathode rays, cannot be refracted in the slightest degree. Light rays always are, and must be, from their very nature as wave motions. Niewenglowski therefore discovered penetrating rays of light capable of passing through a sheet of metal, a substance which anybody would consider opaque—not X-rays, nor cathode rays of a Crookes tube, but light rays of a most remarkable kind. It should be remembered, however, that these penetrating light rays were not given off by the sulphide in its natural condition. It had previously to be exposed to sunlight, whence it derived its energy.
But Becquerel was abreast of the same wave of investigative thought as Niewenglowski. lie says, “For my part, from the day on which I first had knowledge of the discovery of Professor Rontgen, there came to me, too, the idea of seeing whether the property of emitting very penetrating rays was not intimately bound up with phosphorescence.” His [p.358] thought was soon represented concretely; for, taking fragments of various phosphorescent substances, he placed them one after another on a photographic plate enveloped in black paper, and thus gave them an opportunity of telling their secrets by penetrating the paper and affecting the plate beneath.
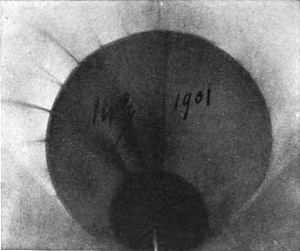
In this his work resembled that of Niewenglowski; but the importance of it is, and the luck of it was, that he experimented with different substances. Out of all the different substances tried, there was one, a substance containing the metal uranium, that had waited æons for this one precious day. For one day of twenty-four hours this substance lay upon a photographic plate enveloped in black paper, and thus, after ages upon ages of waiting, found utterance. This plate was affected. A glance at Fig. 3 will make it evident; and a close examination will reveal the shadow of the copper cross through which the rays had to pass. The plate is obscure, as would be the picture of the approach of dawn; and it is equally significant. It reveals nothing but the presence of penetrating rays.
“Here I am,” said Nature. “Now tell me, am I Niewenglowski's rays?”
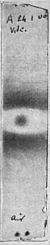
A second experiment (Fig. 4), in which an aluminum medal was placed between the uranium salt and the plate, fairly shouts for an answer to the question, “Am I Niewenglowski's rays?”

“I thought, then,” says Becquerel, “that it was necessary previously to expose the substance to light in order to provoke this penetrating emission, but a short time after I recognized that the emission of the rays was produced spontaneously, even when the substance had been kept completely sheltered from any previous exposure to light.”
This settles the question. Niewenglowski's rays were directly due to the action of the sun upon the substance which emitted them; Becquerel rays arise from a substance whose natural property it is not only to emit them, but, apparently, to manufacture them. It may be stated here that since this discovery the rays emitted by this particular fragment of uranium compound have shown no signs whatever of diminution. They are apparently a permanent property of this form of matter. Furthermore, it was soon seen to be a matter of indifference what uranium substance was employed. Any substance containing uranium gave [p.359] off the rays. Metallic uranium itself, obtained in Moissan's electric furnace, gave out more rays than any of its compounds. More than that, the emission of the rays turned out to be altogether independent even of phosphorescence. Uranium bodies, whether phosphorescent or not, emitted rays. Here, then, was no stored-up, transformed sunlight, such as Niewenglowski's rays, but penetrating, continuous emissions from a substance having no relation to light. The emission of rays capable of passing straight through copper from a chemical substance in its normal condition constitutes to us a new property of matter,— a new thing in nature! So, as Becquerel stood in his laboratory that night, with this thought in his mind and the plate in his hand, he appears sharply silhouetted against the background of the ages; he is comparable with that Theophrastus who, two thousand years ago, rubbed a piece of amber on his coat sleeve and noticed that it attracted bits of paper, unknowing that his bit of amber was equal to the lamp of Aladdin,—or to that paleolithic savage who, the first of all men, noticed the attractive powers of loadstone. New properties of matter are not so common that their significance can be exaggerated. This new property of matter was called radio-activity, and as such it takes its place beside magnetism, electricity, light, and heat.
Radio-activity, a new property of matter, had been discovered, but whence its source? “The metal uranium itself,” you say, “since it gives off the rays.” Yes; but still a doubt — a little, tiny doubt —remained.
Was it not possible that the power of emitting rays, the radio-activity, was due to some small impurity present in the uranium? That doubt was the key which unlocked the door to a roomful of other discoveries.
It arose in the minds of two investigators who had been interested observers of Becquerel's work, M. Pierre Curie, Professor of Physics in the School of Physics and Industrial Chemistry at Paris, and Madame Sklodowska Curie, his wife. They resolved to investigate the ray-emitting power of pitchblende, the parent substance from which all uranium is extracted. To their gratification they discovered that selected specimens of pitchblende possessed a radioactivity four times greater than metallic uranium itself.
Nature never insults us by caprice, and consequently we find the Curies saying: “It becomes, then, very probable that if pitchblende has so strong an activity it is because the mineral contains, in small quantities, a substance wonderfully radioactive, different from uranium or any of the simple bodies actually known. We proposed to ourselves to extract this substance from pitchblende, and we have, in fact, been able to prove that it is [p.359] possible, by the methods of ordinary chemical analysis, to extract from pitchblende substances of which the radioactivity is in the neighborhood of 100,000 times greater than that of metallic uranium.”
In this simple manner did the Curies announce their discovery of three new elements with transcendent ray-emitting powers—radium, polonium, actinium. Of these three strangers, radium has been selected for purposes of research into the character of Becquerel rays because it was most easily obtained. Its discovery, with its ray-emitting power 100,000 times greater than uranium, placed in the hands of Becquerel a mighty engine of research for determining the properties of his rays.
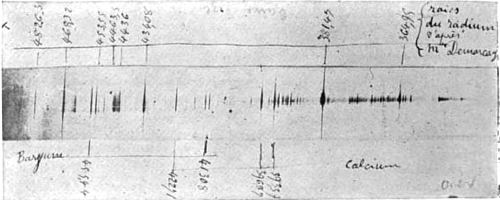
Radium has never been isolated. As a free element it has never been seen, never been touched, never been handled, as gold and iron may be, but it is manifest in the properties of its compounds. It has been studied only in combination with other elements. We know that it exists as an element different from every other body in nature solely and completely through the fact that every element has its own sign-manual, or spectrum, by means of which it signifies its existence, whether it is found in the sun, the stars, or the laboratory. Fig. 5 is the spectrum of radium as obtained by M. Demarcay from a small quantity of chemically pure radium chloride provided by Professor Curie. The lines numbered at the top of the picture are caused by no other known element on the earth or in the heavens. Therefore radium is a new element. The amount of radium in pitchblende is less than one ten-millionth per cent., and the quantities of the much rarer polonium and actinium are literally infinitesimal.
Considering only the cost of the pitchblende from which it is extracted, the value of the radium would be at least $10,000 a gram. As a matter of fact, less than a gram exists. M. Curie possesses about two to three hundredths of a gram of chemically pure radium chloride, which was utilized by M. Demarcay in obtaining the spectrum, and about three-tenths of a gram of a comparatively pure product containing barium chloride.
With the sample of impure radium chloride generously provided by M. and Madame Curie, Becquerel proceeded with the [p.361] study of the properties of his rays. Their surpassing power of penetrating matter generally considered opaque led to their discovery, as we have seen, and it was therefore one of the first properties to be investigated. It soon became evident that this power was quite independent of the kind of matter through which they passed. It was influenced only by the density of the substance interposed. Aluminum, for example, being light in weight, is to Becquerel rays what glass is to light— comparatively transparent. Lead, on the contrary, being heavy, is comparatively opaque.
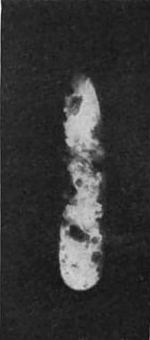
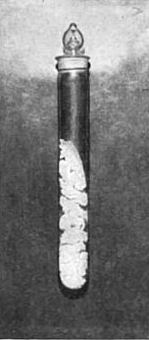
[To obtain the wonderful photographs shown In Fig. 6 (a and b), less than 3/100 of a gram of chemically pure radium chloride was procured by M. Curie. The value of a gram of radium would be $10,000 or more. Less than a gram exists.]
In the power to take radiographs, Becquerel rays resemble X-rays. Many substances when they are exposed to Becquerel rays shine in the dark—that is, they phosphoresce. The diamond and the ruby shine out vividly on being held up in the invisible rays emitted by a pinch of chloride of radium. So do fluor-spar, calcium sulphide, barium platino-cyanide, and many others. So powerful is the phosphorescence caused by Becquerel rays that if a tube of radium chloride be held to the forehead, and the experimenter close his eyes, he will still see light. The retina itself becomes phosphorescent. They even react upon the radium substance itself, so that it too becomes luminous, and shines vividly with a light which, since the discovery of radium, has shown no shadow of variableness. Becquerel rays will photograph the substance which emits them. Fig. 6 (a) is a picture of some radium chloride photographed by daylight, and Fig. 6 (b) shows radium chloride photographed in the dark by its own light—a life-size [p.362] portrait of the only radium chloride in the world executed by itself! They are strange things, then, these Becquerel rays. The light which took the picture shone when the morning of creation broke, and will shine with the dawn of the last day of reckoning; for Becquerel rays are a property of the atom of the substance, and are therefore indestructible. It is a matter of indifference what physical stress is brought to bear, or what chemical transformation is effected. The light will shine, undiminished and undiminishable,— in the gram, a soft radiance; in the pound, if we could get it, a new sun. The physiological effect of Becquerel rays is most intense—almost incredible. A pinch of radium salt, contained in a sealed glass tube, was placed in a card-board box, which was then tied to the sleeve of Professor Curie for an hour and a half. An intense inflammation resulted, followed by a suppurating sore which took more than three months to heal.
[p.363] Professor Becquerel, as he went about his work one day, chanced to carry a sealed glass tube of radium salt in his pocket, placed there for convenience. He was sorry; for the sore was painful and most tedious in healing.
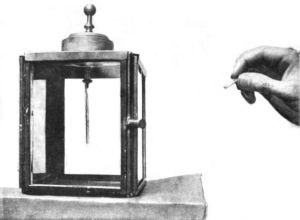
Photographic plates and electrified bodies are widely different. Yet Becquerel discovered, at about the same time, that they were both affected by his rays. A photographic plate was blackened; an electrified body was discharged: either was a detecter of radio-activity. With the discovery of radium, the discharging effect became, of course, exceedingly apparent. Fig. 7 is an electroscope with its little gold leaves spread apart by electrification. On the approach of a glass tube containing a tiny amount of radium chloride, the leaves at once collapse through the discharge of their electrification (Fig. 8). The approach of radium and the discharge of the leaves are simultaneous. Investigation showed that the effect was due to the fact that the rays emitted by the radium spontaneously rendered the air a conductor of electricity, and naturally the electrification of the leaves flew away with as much ease as if they had been touched by a copper wire. As a matter of fact, an electrified body is a more sensitive detecter of radioactivity than a photographic plate.
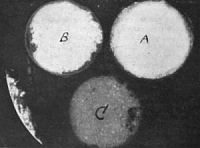
Any substance placed near radium becomes itself a false radium. “We have found,” say the Curies, “that any substance placed in the neighborhood of radium acquires a radioactivity which persists for many hours, and even many days, after the removal of the radium. This induced radio-activity increases with the time during which it is exposed to the action of the radium up to a certain limit. After the radium is removed, it decreases rapidly and tends to disappear. The kind of substance exposed to the action of the radium is almost a matter of indifference. They all acquire a radio-activity of their own.” This fact has been verified over and over again by every experimenter in the field. The zinc, iron, and lead fittings, the air of the laboratory, the water, the clothing of the workers, their very persons, in the presence of radium start into activity and give” out rays comparable to radium in affecting a photographic plate and discharging [p.364] electricity. This becomes very vexatious and disconcerting, and extreme care is necessary to prevent the radium giving out rays altogether misdirected. For days Professor Curie was unable to approach his electrometers, or even enter his laboratory, owing to his acquired radio-activity. These secondary radiations, in the case of zinc, were four times as intense as in ordinary uranium. Moreover, this acquired radio-activity cannot be removed by washing, and the Becquerel rays from radium will impart it even after passing through metal screens. It must be remembered, however, that the radio-activity is only temporary. It vanishes sooner or later upon the removal from the neighborhood of the potent radium. Fig. 9 is a photograph, taken in Professor Curie's laboratory, of phosphorescent action caused by these induced secondary radiations. It was obtained in the following way: Two samples of zinc sulphide, A and B, and a sample of a salt of uranium, C, were placed in card-board pillboxes, and these were laid on a metallic plate, under which was the radium chloride. The metallic plate was quite thick enough to be opaque to the Becquerel rays, but under their influence it gave off secondary radiation, which caused the salts within the pill-boxes to phosphoresce. The photographs were taken from above. They were taken from above. They were caused by the induced light of induced radio-activity.
Becquerel rays cause chemical effects. Emitted from radium, they will discolor paper, cause glass to take a permanent violet tint, turn oxygen into ozone, yellow phosphorus into red phosphorus, mercury perchloride into calomel.
We have learned how Becquerel discovered his rays, we have studied their properties, and we are now face to face with the problem most important of all. What are they? Now Becquerel has discovered in some measure what they are; but before exposing his proofs and results it is necessary to describe one more strange property which he discovered them to possess, and which, as it turned out appears to afford a master-key to their nature.
Becquerel rays are bent by a magnet. Let us follow him while he proves this by one of his characteristic, fecund, simple experiments. Taking a narrow photographic plate enveloped in black paper, he placed it horizontally between the poles of a powerful electro-magnet (Fig. 10). On the black envelope of the plate he then placed a little lead trough containing a small amount of the radium compound. The rays could thus affect the plate only by bending over, for the lead trough is opaque. He [p.365] energized the magnet; then, after a certain time, he reversed the polarity, thinking that, if the rays bent at all, they would then bend in the opposite direction. Fig. 11 is the answer—clear as sunlight. The plate shows two broad bands, proving that the rays must have been curved down to meet it; that there are two bands instead of one proves that reversing the polarity causes the rays to bend in the opposite direction. Becquerel rays are deviable by a magnet.



But are they all equally deviable? Are they homogeneous? Let us follow Becquerel. Upon another plate of the same kind he placed strips of platinum, aluminum, and paper, and at the end of the plate, as before, the little lead trough containing the radium compound. If they were all equally deviable, they would form a line where they bent to meet the plate; if not. they would form a band. After energizing the magnet and developing the plate, he obtained the result shown in Fig. 12. The rays are not equally deviable; they form a broad band, a veritable spectrum of an infinity of radiations unequally deviable. The same plate shows as well that the rays penetrate the screens in this order: the platinum least, the aluminum next, and the paper most of all.
Are they all deviable? Is it not possible that some of them are totally unaffected by the magnet, and do not bend at all? To find out, why not place the narrow photographic plate vertically instead of horizontally between the poles of the magnet? The idea was carried out, and the result is plain beyond all question. The Becquerel rays consist of two distinct kinds of radiation. One kind, A (Fig. 13), is bent by the magnet; the other, B, is totally unaffected by it, and passes undeviatingly on. The print Fig. 14, taken under the magnet, shows still more clearly the existence of two distinct kinds of radiation. Whatever they are, Becquerel rays are a mixture of deviable and undeviable radiations.
| Name of Ray | Cathode Rays | Deviable Becquerel Rays | X-rays | Undeviable Becquerel Rays | Niewen- glowski's Rays |
Ultraviolet Light Rays | Red Light Rays |
| Existence of interference, polarization, reflection, refraction | O | O | O | O | X | X | X |
| Photographic effect | X | X | X | X | X | X | O |
| Excite phosphorescence | X | X | X | X | ? | X | O |
| Render air conductive | X | X | X | X | ? | O | O |
| Penetrate opaque bodies | X | X | X | X | X | O | O |
| Undergo deflection by a magnet | X | X | O | O | ? | O | O |
| Velocity relative to that of light | 1·7 | 12 | 1 | ? | ? | 1 | 1 |
Tabular statement of the properties of the different kind of rays.
X stands for the existence of the property.
O, for its non-existence.
But what are they? Examine the tabular statement [above] of the properties of the different kinds of rays, and you will see this curious fact: The properties of the deviable Becquerel rays are identical with those of the cathode rays of a Crookes tube, and the properties of the undeviable rays are identical with those of the X-rays of Röntgen. Identity of property means identity of nature, and we are therefore forced to conclude that the Becquerel rays from radium are nothing more nor less than a mixture of cathode and X-rays, their progenitors in the history of discovery.
“What an anti-climax!” says the reader. “We started out to study a new property of matter, and here we end up with an old one.”
Not a bit of it. We called the new property of matter radio-activity—not Becquerel rays. “What is the difference?” [p.366] All the difference between a natural intrinsic property and a property of condition. The light of an arc-lamp is a property of condition; suppose you found, deep in the earth, a substance blazing forever with a light as great, that would be a natural intrinsic property— and a very curious one—radio-activity. So with the cathode and X rays. They arise from a Crookes tube, a mechanism which is the complicated result of centuries of thought; they are a property of condition. The Becquerel rays from radium, on the contrary, arise from a substance dug out of the ground which emits them, apparently, forever and forever, as it has emitted them through the countless centuries of the past, without any extrinsic influence. It is their natural intrinsic property—a new property of matter—radio-activity.
The cathode rays are streams of material particles. These particles are projected from radium with a velocity anywhere from sixty millions to ninety millions of miles per second. They fly out laden with electricity, and hence naturally enough discharge an electroscope. They are so small that the atoms of the chemist are giants in comparison. Since these particles flying off from radium are decomposed atoms, their properties are not the properties of iron, or gold, or copper, but the properties of matter in general. These particles, or corpuscles, as they are called, appear to be the primary atoms of some parent form of matter out of which the elements, as we know them, have been evolved. It is interesting, in this connection, to recall the words of Huxley, written long ago, before Becquerel rays had entered into the dreams of the wildest speculator. “It seems safe to assume,” he wrote, “that the hypothesis of the evolution of the elements from a primitive form of matter will in the future play no less a part in the history of science than the atomic hypothesis, which, to begin with, had no greater, if as great, an empirical foundation.” These words were written with the prescience of a master.

Possibly the most interesting thought in all the strange, eventful history of these interesting bodies is the question of their energy. Whence does it come? It is suggested by Madame Curie that the radium receives its energy from, and responds to, radiations which traverse all space, much as some article of bric-à-brac in a room will vibrate responsively to a certain tone of the piano. This may be. Heaven only knows. One thing we do know—space is all aquiver with waves of radiant energy, ranging in length from many feet to a size infinitesimally small. To only a few of these are our bodily senses fitted to correspond, or our mechanisms to detect. Waves of radiant energy constitute what has been called “the harp of life.” We vibrate in sympathy with a few strings here and there— with the tiny X-rays, actinic waves, light waves, heat waves, in the treble, and the huge electro-magnetic waves of Hertz and Marconi, and the grand air waves of sound, in the bass; but there are great spaces, numberless strings, an infinity of possible radiations, to which we are deaf —stone-deaf. Some day, a thousand years hence, we shall know the full sweep of this magnificent harmony, and with it we shall vibrate in accord with the Master Musician of it all.

- Science Quotes by Henri Becquerel.
- 15 Dec - short biography, births, deaths and events on date of Becquerel's birth.
- Becquerel Rays - article by J.J. Thomson from Harper's Magazine (Aug 1902).
- Radioactivity: A History of a Mysterious Science, by Marjorie C. Malley. - book suggestion.