 (source)
(source)
|
Charles Martin Hall
(6 Dec 1863 - 27 Dec 1914)
American chemist who invented (1886) an inexpensive electro-chemical method to extract aluminium from its ore. The process quickly enabled commercial production of this metal, at prices competitive with steel and copper. Aluminium, once a precious metal used for fine jewelry, by 1914 was down to 18 cents a pound.
|
THE AMERICAN CREATOR OF THE ALUMINUM AGE
CHARLES MARTIN HALL, INDUSTRIAL CHEMIST, WHOSE RESEARCHES TRANSFORMED ALUMINUM FROM A PRECIOUS METAL TO ONE OF COMMON COMMERCIAL USE—HOW IT IS REPLACING STEEL AND COPPER AND WHY IT MAY GIVE THE NAME TO A NEW ERA IN INDUSTRIAL ARTS
by JOHN M. OSKISON
from The World’s Work (1914)

FROM A PHOTOGRAPH THAT WAS TAKEN ABOUT THE TIME HE INVENTED THE ELECTRICAL PROCESS OF EXTRACTING PURE ALUMINUM FROM CLAY WHICH HAS MADE POSSIBLE THE COMMERCIAL USE OF THE METAL
[p.438]
THE HISTORY OF ALUMINUMAS TOLD BY ITS PRICE |
||
| 1855 . . . . . . . . | $90.00 | a lb |
| 1857 . . . . . . . . | 22.50 | “ “ |
| 1862 . . . . . . . . | 12.00 | “ “ |
| 1886 . . . . . . . . | 12.00 | “ “ |
| 1888 . . . . . . . . | 5.00 | “ “ |
| 1914 . . . . . . . . | .18 | “ “ |
IT IS not boasting in these days to say that we are capable of creating a new metallic “Age” within two generations—almost within one lifetime. We have done it; and the credit goes to the workers in industrial laboratories. Consider the story of Charles Martin Hall, a modest, patient investigator, and of his work with aluminum.
Aluminum is the most abundant metal. Deville, an early French experimenter, declared that every clay bank is a deposit of the metallic earth from which it is extracted; last year, four states—Alabama, Arkansas, Georgia, and Tennessee—contributed the 210,000 tons of bauxite (the ore from which aluminum is taken) that was mined for manufacture in this country.
Knowledge of how to use the metal is growing. In 1908, our production was 11 million pounds, and the latest figures show that last year we imported 27 million pounds to supplement a home production of 65,500,000 pounds. It is cheap enough already to have become a real competitor of copper and iron; and to-day manufacturing facilities in the United States are being increased at a rate which foretells such a vast development in the use of aluminum within a few years as will make the present output seem insignificant.
Three and a half years ago, when Charles Martin Hall received from the chemists of America the Perkin medal, the presentation was made by Dr. C. F. Chandler, of Columbia University. Full of years and honors himself, Dr. Chandler reviewed his own connection with the history of aluminum and explained his life-long interest in it. In one way, Dr. Chandler’s experience covers the whole history of the period in which the metal has been known.
As a student of 18, in 1854, young Chandler heard Wöhler’s account of his discovery of aluminum in 1827. Wöhler’s lecture opened to the vision of the student a new element, a still-fresh miracle of the scientist’s laboratory, its possibilities all unrealized. Later, as teacher and investigator himself, Dr. Chandler followed the patient, intelligent work of Mr. Hall, and saw it flower into an actual new industrial epoch.
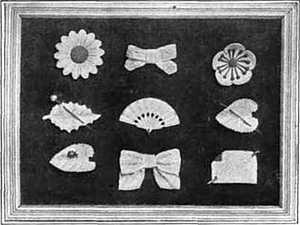
ALMOST THE ONLY USE OF THE METAL FORTY-FIVE YEARS AGO. WHEN IT WAS WORTH $12 A POUND
A hundred years before young Chandler heard Wöhler's lecture, a chemist named Margraf found a peculiar chemical earth in alum. He thought it contained a metal, and he called it “alumina.” His researches, however, brought to light nothing more than the name. Half a century later, in 1807, Sir Humphry Davy tried reducing this “alumina,” and succeeded in producing an alloy of metals—but still the metal aluminum was unborn, and it was twenty years later that [p.439] Wöhler tackled the problem. With potassium, Wöhler was enabled to reduce aluminum chloride to a gray metallic powder. The first bit of this metal he ever saw was shown to young Chandler in 1855 by a chemist named Rose; so precious was it that a pound was worth $90.
ALUMINUM AS JEWELRY
Louis Napoleon became interested in the story of the gray powder and the possibilities of its conversion into a new sort of metal. He subsidized Henri St. Claire Deville, who under took to establish a factory for the production of aluminum. That was in 1854. Up to 1888, Deville’s plant continued to turn out the metal and its manufactures, reaching a maximum production of 5,000 pounds a year. This was practically all converted into small fancy articles—rings, brooches, statuettes, thimbles, souvenir bars, and wire coils. In Paris, in 1869, Dr. Chandler paid $9 for a four-ounce basket that was made of aluminum.
Between Deville and Hall in the history of the development of the aluminum industry came Hamilton Y. Castner, who studied under Dr. Chandler. Castner’s discovery was that caustic soda and iron carbide could be used in making sodium instead of the far more expensive carbonate of soda and carbon employed by Deville. Still, as three pounds of sodium were required, in the reducing process, for the production of one pound of aluminum, and as Castner's sodium cost 25 cents a pound, he never reached a solution of the problem of making the metal cheap enough for general use. By 1889, however, he was turning out 500 pounds a day and selling it for $4 a pound.
Very briefly, that was Dr. Chandler’s story of the development of aluminum up to 1886, when Charles Martin Hall, a young American graduate of Oberlin College, discovered an entirely new process for making the metal.

AN ALUMINUM AIRSHIP
THE FRAME OF THE HUGE ZEPPELIN DIRIGIBLE BALLOONS CAN BE MADE OF ALUMINUM BECAUSE THE COST OF THE METAL IS NOW ONLY 18 CENTS A POUND
ITS MARVELOUS PROPERTIES

THE FRAME OF THE HUGE ZEPPELIN DIRIGIBLE BALLOONS CAN BE MADE OF ALUMINUM BECAUSE THE COST OF THE METAL IS NOW ONLY 18 CENTS A POUND
Through the minds of alert industrial chemists, of course, had been running the stories of this wonderful new metal whose specific gravity was only two and a half times that of water, which when hammered and rolled became as hard as iron and lighter than glass, and which did not rust or tarnish. Dr. Chandler, for one, had been actively interested in it since 1854, and many other experimenters were working at it.

THE LITTLE BASKET, WHICH WEIGHS ONLY FOUR OUNCES, COST $9 WHEN PROF. C. F. CHANDLER, OF COLUMBIA UNIVERSITY, BOUGHT IT IN PARIS FORTY-FIVE YEARS AGO
Charles Hall’s father, who was a minister, had in his library a book he had studied—a battered text book of chemistry published in 1841. This came into his son’s hands while he was in school. What that author knew about aluminum was contained in this paragraph:
“The metal may be obtained by heating chloride of aluminum with potassium in a covered platinum or porcelain crucible and dissolving out the salt with water. As thus prepared, it is a gray powder similar to platinum, but when rubbed in a mortar exhibits a distinctly metallic lustre. Fuses at a higher temperature than cast iron, and in this state is a conductor of electricity but a non-conductor when cold.”
In school, the boy studied chemistry, but when he entered Oberlin College he did not specialize in that science. His course was the usual classical course that is elected by most students. He was one of the serious-minded students of the college who “ate up his work;” yet beyond the boundaries of his own class work his mind went searching for new problems. He read with unusual interest Deville’s statement that though every clay bank was a mine of aluminum, the metal was as costly as silver. Surely, here was something worth looking into.

A HINT FROM AN OLD TEXT BOOK
Graduated in the class of 1885, young Hall found his mind full of scientific curiosity concerning aluminum; from Professor Jewett he secured permission to use the college laboratory during the summer, and he hunted up his father’s old text book again. He re-read what it said about aluminum; perhaps he got a hint from the book’s statement that when fused (that is, heated until it is in a liquid state) the metal is a conductor of electricity. Anyhow, his experiments during that summer and fall were concerned with the effect of electricity on the metallic earth [p.440] “alumina.” Fascinated by the problem, the young man went after the secret of cheap aluminum in earnest.
First of all, he found that he needed a cheap, practicable method of reducing the metallic earth “alumina” to a fluid condition so that an electric current would flow through it. He wanted an anhydrous solvent (my dictionary says that anhydrous means “destitute of water”)—something he could mix with “alumina” and get a fused mass at a reasonable temperature. The summer and fall ran into winter, and the young experimenter still sought a suitable solvent.
In the following February, of 1886, he found it; it was cryolite, a mineral used in the manufacture of soda and glass. Cryolite fused readily at a low temperature, and when fused it absorbed more than 25 per cent, of its own weight of the white powder of “alumina” while still remaining clear and limpid. As young Hall dropped “alumina” into the fused bath of cryolite, he was delighted to see that it dissolved like sugar or salt in boiling water.
A THEORY THAT WORKED

He had made his big discovery; and he was just past the age of twenty-two!
Through that clear, limpid bath of fused cryolite and “alumina” he was ready to shoot an electric current and precipitate aluminum. From Professor Jewett he borrowed a battery and made the first trial. It failed, and he reasoned that it failed because he had used a clay crucible; when he substituted a carbon-lined crucible, he got some globules of aluminum; his theory, a revolutionary one among the workers in that field, had been proved sound scientifically.
The task of adapting his discovery commercially remained—a far longer, if not more formidable, job than he had already finished. It was in this further working out of the process and the transformation of the laboratory miracle into an industrial commonplace that Mr. Hall's mettle was tested.
He was very young; his chemical training had been scant—and men with capital were skeptical. Technical difficulties arose. He found that the carbon poles which carried the electric current into the fused mass burned up quickly. Platinum poles resisted the heat successfully, but Mr. Hall knew that this material was far too expensive to use. With certain adjustments of the fused mixture, however, he was able to use cheaper copper poles. It seemed a solution, yet after a year of experimenting he abandoned the mixture as an unprofitable one.
For a long time it seemed that the Hall electrolytic process was fated to join the long list of inventions which are ideal in theory but are damned as impractical. Of course, Mr. Hall believed that the difficulties could be overcome, but he had indifferent luck in making others agree with him. For three years, approximately, he had to fight the handicaps with little encouragement.
JUDGE TAFT’S SUPPORTING DECISION
Mr. Hall’s discovery was made in February, 1886; in July, he applied for a patent on his process; the basic patent was granted in April, 1889. Later, additional patents, perfecting the process, were granted to him. He did not escape the fate of most American inventors, though he kept his patent application in the Patent Office nearly three years; his patents were attacked, and he had to fight them through the courts. In the United States Circuit Court for the Northern District of Ohio his claims were sustained by Judge William H. Taft. In his decision Judge Taft wrote:
“Hall was a pioneer, and is entitled to the advantages which that fact gives him in the patent laws.”
After applying for his basic patent, Mr. Hall set out to secure financial backing. Through his brother, he succeeded in interesting some Boston men. They stuck to him until October, 1886, and then quit because they could not see any profitable future for the process. In Cleveland, Mr. Hall sought the president of one of the biggest chemical manufacturing companies in the country. This man showed a real interest in the process; but from what Mr. Hall has said, his interest was [p.441] too strictly financial. At Lockport, N. Y.. was an electric smelting concern which was turning out aluminum bronze. Mr. Hall made a deal by which this company agreed to try his process. For a year—from July, 1887, to July, 1888—the company gave Mr. Hall and his process opportunity to make good. But in that year the process did not take advantage of the opportunity.
Mr. Hall by this time thought that he had found out the reason for the failure. He explained to the men at Lockport that if he enlarged the ‘‘bath” (which contained the fused mixture through which the electric current passed) and thus moved the poles of his current farther apart, he could probably prevent the “clogging” of the bath with the worthless black precipitate which had appeared. They listened, but were unconvinced; they said good-bye to him and his process.
With the help of his brother, Mr. Hall set out to find new backing.
“Let’s go to Pittsburg,” said the brother. “There’s a city that is looking for new ideas.” It was true that Pittsburg in 1888 was the nursery of fresh industrial impulses as well as the home of vigorous giants of manufacture. The capitalists of Pittsburg were used to seeing bread that was cast upon the waters of industrial invention come back well buttered.
Within a surprisingly short time after going to Pittsburg, Mr. Hall got the backing he sought. The Pittsburg Reduction Company was formed, and a small plant was secured at Kensington, near that city. By November of 1888 it was turning out fifty pounds a day of [p.442] aluminum, and the company was selling it for $2 a pound. In the plant at Kensington, using an enlarged “bath,” the old trouble with clogging disappeared—just as Mr. Hall had predicted it would.
At the plant at Kensington, too, the inventor made the pleasing discovery which he had predicted, that no external heat was needed to keep the bath fused—the electric current passing through the mixture was sufficient.
Beginning in a factory with fifty available horse-power and with a daily production of fifty pounds, the company grew rapidly, in 1890, Castner, who was using his caustic soda and iron carbide process in England to turn out 500 pounds a day for S4 a pound, retired from the field; Deville had quit two years before.
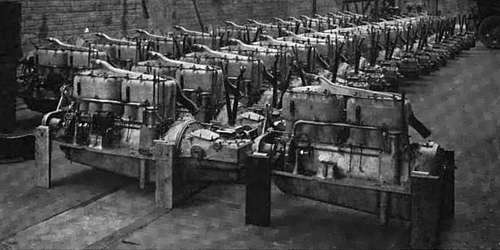
THE CRANK CASES, TRANSMISSION CASES, AND WATER PIPES OF THESE AUTOMOBILE POWER PLANTS ARE MADE OF THE METAL BECAUSE IT LESSENS DEAD WEIGHT
Twenty-five years covers the history of the Hall process; his company has grown rapidly and steadily, for the bankers in Pittsburg who got behind him never lost faith and furnished the money for more and bigger plants. At the time the Perkin medal was given him, Mr. Hall came from his home at Niagara Falls, where he has lived and worked ever since the Falls were harnessed for the generation of electric power; his company was the first user of that power. At the beginning of 1911, the company’s three factories were using 140,000 horse-power of electric current and turning out 40,000,000 pounds of aluminum a year; the price had gone down to 22 cents a pound. Mr. Hall and his associates are the biggest users of electricity among the great electro-chemical works of the world.

ONE AUTOMOBILE FACTORY NOW USES MORE ALUMINUM EVERY YEAR THAN THE WHOLE WORLD PRODUCED IN THAT TIME TEN YEARS AGO
If this were strictly the story of the discovery and development of the electrolytic process for the manufacture of [p.443] aluminum, the name of a brilliant Frenchman, Paul T. L. Héroult, would be coupled throughout with that of Mr. Hall. In Europe, Mr. Hall is all but unknown, and M. Héroult, who died last May in Paris, was recognized as the discoverer of the [p.444] process. The case of Hall and Héroult is one of the authentic cases of simultaneous discovery.
Recall that Mr. Hall got his definite results in February, 1886. In April, 1886, M. Héroult attracted the attention of the chemical world by discovering, in France, the identical process. He, too, was a brilliant young worker, for he was born in the same year as Mr. Hall. About the time Mr. Hall’s company was getting started at Pittsburg, M. Héroult came to America to take charge of the exploitation of a process with various steel and metal companies.
Much of his active career was identified with this country.
M. Héroult was present at the meeting of chemists at which Mr. Hall was honored, and he made a little speech.
It was different from the sort of speech you would expect to hear on such an occasion from an expert in chemistry. In it he told, with great charm and humor, something about his own discovery of the electrolytic process for aluminum.
He recalled that at the callow age when life seems hardly worth living, when the yearnings of Kipling’s soldier in “Mandalay” to be shipped “somewheres East of Suez” strike a young man as an imperative call—that is, about the age of twenty—M. Héroult and his very dear friend and comrade (later his partner in his big manufacturing enterprise) found themselves in the streets of Paris “dead broke.” Restlessly, they had pursued pleasure—also, with no regard for cost. Everything pawnable had gone to the money lenders, and the question was where to raise more francs.
“Think, my friend!” urged young Héroult; and after a decent interval the friend produced an inspiration: His aunt owned a souvenir bar of aluminum, six inches long, which had come from the works of Deville. It ought to be very valuable; he would “borrow” it, and no doubt the pawnbroker would advance them a reasonable sum on it. Excellent!
They secured the bar of aluminum and proffered it to the pawnbroker. Without touching it, he asked:
“Is that silver?” “No,” they told him in chorus, “it’s better—aluminum!” “Aluminum—what’s that?” asked the pawnbroker, and picked it up. “Why, it’s hollow!”
“No, no,” objected the two young spendthrifts, “it’s aluminum, and it’s worth $2 a pound.” At that, the pawnbroker weighed the bar in his hand and regarded it long and prayerfully.
“Well,” he said finally, “I’ll give you 40 cents on it.” Said M. Héroult to the assembled chemists who listened to his little talk: “On a hot summer day it was better than nothing! We took the money with the firm intention of redeeming the stick, which we never did. Maybe that is one of the reasons why, later on, I had to make good and replace it.”
In Europe, M. Héroult certainly did make good with his process. Within three years it was generally adopted over there; and the production of aluminum in Europe has increased even more rapidly [p.445] than in this country. In 1911, it had risen to 100,000,000 pounds a year, and its market price had dropped to 20 cents a pound.

LEFT: F. WÖHLER, WHO, IN 1827, BY CHEMICAL MEANS FIRST OBTAINED ALUMINUM IN A METALLIC STATE. RIGHT: HAMILTON Y. CASTNER, AN AMERICAN WHO DISCOVERED A COMPARATIVELY CHEAP CHEMICAL METHOD OF PRODUCING ALUMINUM
M. Héroult concluded his little talk with a prophecy: within ten or fifteen years, he said, aluminum will be used as extensively as copper; perhaps it will have fixed its name upon a new age of metal—as iron named the Iron Age.
Away back in the mists of time began the centuries-long Stone Age; then men learned to fashion implements from an alloy of tin and copper, and the bronze age began its long reign; there came the Iron Age to take its toll of the centuries and give its marvelous impetus to civilization—but we begin to suspect that we have about exhausted the possibilities of the Iron Age. We are outgrowing iron—it won’t do; for one thing, it won’t do for the equipment of our air-craft—and aluminum will do.
As an alloy, aluminum is creeping more and more into the substance of steel; it is replacing copper as a conductor of electricity; it is used to tame refractory oxides and reduce them to carbon-free metals; it is a welding agent as Dr. Hans Goldschmidt employs it in his alumino-thermic process. Dr. Goldschmidt now supplies the world with chromium, manganese, cobalt, and alloys of iron with chromium, vanadium, molybdenum, and titanium, all carbon free. Into the world’s millions of automobiles and thousands of airships, aluminum will enter wherever strength and lightness must be combined; and in the kitchen (no, this isn’t an anticlimax!) it is replacing iron and tin.
For America, Charles Martin Hall—a round-headed, modest, quiet, boyish-looking worker—opened the door upon the new age of aluminum. To Europe, Paul Héroult revealed it. There is credit enough in the achievement for both; and until Héroult died they shared it like gallant rivals.

Aluminum rolling mill and pot room buildings. Viewed from Canadian side of the gorge, down river from Niagara Falls. (source)
- Science Quotes by Charles Martin Hall.
- 6 Dec - short biography, births, deaths and events on date of Hall's birth.
- Charles Hall: Invention of the Aluminum Process - Perkin Medal Award Speech (1911)
- Properties of Aluminum - by Charles Hall, from Western Electrician (1891).
- Aluminium History - from Scientific American Supplement (6 Mar 1886)
- Made of Aluminum: A Life of Charles Martin Hall, by Rosamond McPherson Young. - book suggestion.





