(source)
(source)
|
John Tyndall
(2 Aug 1820 - 4 Dec 1893)
Irish physicist who demonstrated why the sky is blue. He wrote on diverse topics, including crystals, glaciers and radiation. His studies also included spontaneous generation, the germ theory of disease and ozone.
|
On Matter and Force.
By Professor Tyndall, F.R.S.
[p.256] This lecture was not addressed to members of the British Association, but to working men. of whom nearly 3.000 were present, the large Kinnaird Hall being crowded to suffocation. To give the lecture at length would occupy more pages than can be well spared. I cannot refrain, however, from quoting some extracts from one of the most eloquent addresses which this gifted orator has ever delivered. The subject matter of the lecture offered no special interest to those who are in the habit of listening to this philosopher in his home—the Royal Institution, but not a word of experiment was lost upon the auditors, who testified their admiration of the noble and high-toned sentiments by silent rapt attention, and of the brilliant experiments by enthusiastic applause, occasionally so prolonged that the lecturer was obliged to beg them to moderate their cheering, or he should be obliged to extend the hour and a half allotted to him to two hours. Professor Tyndall’s language is always elevated in tone, and his speculations bold and fearless; and those who listened to the climax of tumultuous applause which followed his splendid peroration, looked in vain for any evidence of that intolerance of free-thought which is supposed to be a national characteristic north of the Tweed.
Professor Tyndall, commenced by remarking on the avidity with which the working men of London seized the opportunities afforded them by the evening lectures delivered every year by the Professors in the Royal School of Mines. It was a noteworthy fact that these lectures were but rarely of a character which could help the artisan in his daily pursuits. It was a pure desire for knowledge, as a thing good in itself, and without regard to its practical application which animated these men.
“They wish to know,” he continued, “more of the wonderful universe around them; their minds hunger for this knowledge as naturally as their bodies hunger for food, and they come to us to satisfy this intellectual want. It is easily argued as a plea for science that it affords great material benefits. So it does. No doubt of it. Without science your Dundee would be a very small place indeed. But still the scientific discoverers—those high priests, so to say, of Science—they are, I assure you, in this work very rarely actuated by a desire for practical application at all. Take that great man whose name I can hardly trust my lips to utter—that man whom I loved—and who has lately gone from this earth —that man Faraday. That man—a poor book-binder’s apprentice—has done more for practical science than dozens of your practical men added together; and he has done it without ever caring to gain a shilling by it. He did it because he loved science; and I say it behoves the Legislature to know that the real workers in science are not those who are always trying to turn it to a practical account They love science for itself, and their desire is to understand and know all the phenomena of this glorious universe.
Whether it be a consequence of long-continued development, or whether it be an endowment conferred once for all on man at his creation, we find him here gifted with a mind, curious to know the cause of things, and surrounded by things which excite its questionings, and raise the desire for an explanation. It is related of a young Prince of one of the Pacific Islands, that when he first saw himself in a looking-glass, he ran round the glass to see who was standing at the back. He wished to know the cause of what he saw. And thus it is with the general human intellect when it regards and ponders the phenomena of the external world. It wishes to know the causes and connections of these phenomena. What is the sun, what is the earth, what should we see if we came to the edge of the earth and looked over? What is the meaning of thunder and lightning, of hail, rain, storm. and snow? Such questions early presented themselves to men. and by and by it was discovered that this desire for knowledge was not implanted in vain. After many trials it became evident that man possessed the power of solving such questions—that within certain limits the secret of the universe was open to the human understanding. It was found that the mind of man had the power of penetrating far beyond the boundaries of his five senses; that the things which are seen in the material world depend for their action upon things unseen; in short that besides the phenomena which address the senses, there are laws and principles and processes which do not address the senses, but which must be, and can be, spiritually discerned.
Now, there are two thing which form, so to say. the substance of all scientific thought. The entire play of the scientific intellect is confined to the combination and resolution of the ideas of matter and force. Newton, it is said, saw an apple fall. To the common mind this presented no difficulty and excited no question. Not so with Newton. He observed the fact; but one side of his great intellectual nature was left unsatisfied by the mere act of observation. He sought after the principle which ruled the fact Whether this anecdote be true or not, it illustrates the fact that the ordinary operations of nature, which most people take for granted as perfectly plain and simple, are often those which most puzzle the scientific man. To the conception of the matter of the apple Newton added that of the force that moved it. The falling of the apple was due to an attraction exerted mutually between the apple and the earth. He applied the idea of this force to suns and planets and moons, and showed that all their motions were necessary consequences of the action of this force of attraction. He proved that the planetary motions were what observation made them to be, because every particle of matter in the solar system attracts every other particle by a force which varies as the inverse square of the distance between the particles. He showed that the moon fell towards the earth, and that the planets fell towards the sun, through the operation of the same force that pulls an apple from its tree. And this all-pervading force, the conception of which was necessary to Newton’s intellectual peace, is called the force of gravitation. All force may be ultimately reduced to a push or a pull in a straight line; but its manifestations are various, and sometimes so complex as entirely to disguise its elementary constituents.
Long thinking and experimenting on the materials which compose our world have led philosophers to conclude that [p.257] matter is composed of atoms from which, whether separate or in combination, the whole material world is built up. The air we breathe, for example, is mainly a mixture of the atoms of two distinct substances, called oxygen and nitrogen. The water we drink is also composed of two distinct substances, called oxygen and hydrogen. But it differs from the air in this particular, that in water the oxygen and hydrogen are not mechanically mixed, but chemically combined. In fact, the atoms of oxygen and those of hydrogen exert enormous attractions on each other, so that when brought into sufficient proximity they push together with an almost incredible force to form a chemical compound.
One consequence of the clashing together of the atoms is the development of a great amount of heat. What is this best? How are we to figure it before our minds? I do not despair of being able to give you a tolerably distinct answer to this question. Here are two ivory balls suspended from the same point of support by two short strings. I draw them thus apart and then liberate them. They clash together, but, by virtue of their elasticity, they quickly recoil from each other, and a sharp vibratory rattle succeeds their collision. This experiment will enable you to figure to your mind a pair of dashing atoms. We have, in the first place, a motion of the one atom towards the other—a motion of translation, as it is usually called. But when the atoms come sufficiently near each other, elastic repulsion sets in, the motion of translation is stopped and converted into a motion of vibration. To this vibratory motion we give the name of heat. Thus, three things are to be kept before the mind—first, the atoms themselves; secondly, the force with which they attract each other; and thirdly, the motion consequent on the exercise of that force. This motion must be figured first as a motion of translation, and then as a motion of vibration; and it is not until the motion reaches the vibratory stage that we give it the name of heat. It is this motion imparted to the nerves that produces the sensation of heat.
It would be useless to attempt a more detailed description of this molecular motion. After the atoms have been thrown into this state of agitation, very complicated motions must ensue from their incessant collision. There must be a wild whirling about of the molecules. For some time after the art of combination, this motion is so violent as to prevent the molecules from coming together. The water is maintained for a time in a state of vapour; but as the vapour cools, or, in other words, loses its motion, the water molecules coalesce to form a liquid. And now we are approaching a new and wonderful display of force. No one who had only seen water in its vaporous or liquid form could imagine the existence of the forces to which I am now about to refer; for, as long as the substance remains in a liquid or vaporous condition, the play of these forces is masked by the agitation kept up by the heat among the molecules. But let the heat be gradually withdrawn, the antagonist to their union being removed, the molecules begin to form new combinations. Like the particles of iron in our magnetic experiment, the water molecules arc endowed with attractive and repulsive poles, and they arrange themselves together in accordance with these attractions and repulsions. Solid crystals of water are thus formed, to which we give the familiar name of ice. To the eye of science, these ice crystals are as precious as the diamond—as purely formed, as delicately built. Where no disturbing causes intervene, there is no disorder in this crystalline architecture. By their own structural power molecules build themselves on to molecules with a precision infinitely greater than that attainable by the hands of man. We are apt to overlook the wonderful when it becomes common. Imagine the bricks and stones of this town of Dundee endowed with locomotive power. Imagine them attracting and repelling each other, and arranging themselves in consequence of these attractions and repulsions so as to form streets and houses and Kinnaird Halls; would not that be wonderful? No less wonderful is the play of force by which the molecules of water build themselves into the sheets of crystal which roof your ponds and lakes every winter. To use the language of the American poet, Emerson, “the atoms march in tune,” moving to the music of law, which thus renders the commonest substance in nature a miracle of beauty to the mental eye. It is the function of science, not as some think to divest this universe of its wonder and its mystery, That, as in the case here before us, to point out the wonder and the mystery of common things.
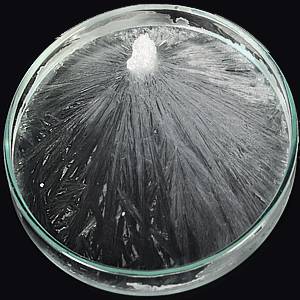
Over a plate of perfectly clean glass I pour a little water in which a crystal has been dissolved. A film of the solution clings to the glass; and now I wish to make this film crystallise before your eyes. By means of a microscope and electric lamp, I throw an image of the plate of glass upon the screen. The beam of the lamp, besides illuminating the glass, also heats it; evaporation is thereby promoted, and, at a certain moment, when the solution has become supersaturated, splendid branches of crystals shoot out over the screen. A dozen square feet of surface are now covered by those beautiful forms. Here we have crystalline spears shooting over the screen, feathered right and left by other spears. Molecule thus closes with molecule, until, finally, the whole subsides into crystalline rigidity. I move a new portion of the film into the beam. From distinct nuclei in the middle of the field of view the crystalline spears shoot with magical rapidity in all directions. For a moment the whole film appears to be alive, and now it has sunk into molecular repose. The film of water on a window pane on a frosty morning exhibits effects quite us wonderful as these. Latent in this formless solution, latent in every drop of water lies this marvellous structural power, which only requires the withdrawal of opposing forces to bring it into action. These experiments show that the common matter of our earth—“brute matter,’’ as Dr. Young calls it—when its atoms and molecules are permitted to bring into free play the forces with which they are endowed, arranges itself under the operation of these forces, into forms which rival in beauty those of the vegetable world. And what is the vegetable world itself but the result of the complex play of these molecular forces. Here, as elsewhere throughout nature, if matter moves it is force that moves it, and if a certain structure is produced it is through the operation of the forces with which the atoms and molecules composing the structure are endowed. These atoms and molecules resoluble little magnets with mutually attractive and mutually repellent poles. The attracting poles unite, the repellent poles retreat from each other, and vegetable forms are the final expression of this complicated play of molecular force. In the formation of our lead and silver trees, we needed an agent to wrest the lead and the silver from the acids with which they were combined. A similar agent is required in the vegetable world. The solid matter of which our lead and silver trees were formed was, in the first instance, disguised in a transparent liquid; the solid matter of which our woods and forests are composed is also, for the most part, disguised in a transparent gas, which is mixed in small quantities with the air of our atmosphere. That gas is formed by the union of carbon and oxygen, and is called carbonic acid gas. Two atoms of oxygen and one of carbon unite to form the molecule of carbonic acid which, as I have said, is the material from which wood and vegetable tissues are mainly derived. The carbonic acid of the air being subjected to an action somewhat analogous to that of the electric current in the case of our lead and silver trees, has its carbon liberated and deposited as woody fibre. The watery vapour of the air is subjected to a similar action; its hydrogen is liberated from its oxygen, and lies down side by side with the carbon in the tissues of the tree. The oxygen in both cases is permitted to wander away into the atmosphere. But what is it which thus tears the carbon and the hydrogen from the strong embrace of the oxygen? What is it in nature that plays the part of the electric current in our experiments? The rays of the sun. The leaves of plants absorb both the carbonic acid and the aqueous vapour of the air; these leaves answer to the cells in which our experiments on decomposition by the electric current took place. In the leaves the solar rays decompose both the carbonic acid [p.258] and the water, permitting the oxygen in both cases to escape into the air, and allowing the carbon and the hydrogen to follow the bent of their own forces. And just as the molecular attractions of the silver and the lead found expression in the production of those beautiful branching forms seen in our experiments, so do the molecular attractions of the liberated carbon and hydrogen find expression in the architecture of grasses, plants, and trees.
In the fall of a cataract or the rush of the wind we have an example of mechanical power. In the combinations of chemistry and in the formation of crystals and vegetables we hate examples of molecular power, before proceeding further I should like to make clear to you the present condition of the surface of the globe with reference to power generally. You have learned how the atoms of oxygen and hydrogen rush together to form water. I have not thought it necessary to dwell upon the mighty mechanical energy of their act of combination, but, in passing, I would say that the clashing together of l lb. of hydrogen and 8 lbs. of oxygen to form 9 lbs. of aqueous vapour, is greater than the clash of a weight of 1,000 tons falling from a height of 20 feet against the earth. Now, in order that the atoms of oxygen and hydrogen should rise by their mutual attractions to the velocity corresponding to this enormous mechanical effect, a certain distance must exist between the particles. It is in rushing over this distance that the velocity is attained. This idea of distance between the attracting atoms is of the highest importance in our conception of the system of the world. For the world may be divided into two kinds of matter; or rather the matter of the world may be classified under two distinct heads—namely, of atoms and molecules which have already rushed together and thus satisfied their mutual attractions, arid of atoms and molecules which have not yet rushed together, and whose mutual attractions are, therefore, as yet unsatisfied. Now, as regards motive power, the working of machinery, or the performance of mechanical work generally by means of the materials of the earth’s crust, we are entirely dependent on those atoms and molecules whose attractions are as yet unsatisfied. Those attractions can produce motion, because sufficient distance intervenes between the attracting molecules, and it is this molecular motion that we utilise in our machines. Thus we can get power out of oxygen and hydrogen by the act of their union, but once they are combined, and once the motion consequent on their combination has been expended, no further power can be got out of the mutual attraction of oxygen and hydrogen. Their mutual attractions are then satisfied, and as dynamic agents they are dead.
Now, if we examine the materials of which the earth’s crust is composed, we find them to consist for the most part of substances whose atoms have already closed in chemical union—whose mutual attractions are satisfied. Granite, for instance, is a widely diffused substance; but granite consists, in great part, of silicon, oxygen, potassium, calcium, and aluminium, the atoms of which substances met long ago in chemical combination, and are therefore dead. Limestone is also a widely-diffused substance. It is composed of carbon, oxygen, and a metal called calcium. But the atoms of those substances closed long ago in chemical union, and are therefore dead. And in this way we might go over the whole of the materials of the earth’s crust, and satisfy ourselves that though they were sources of power in ages past, and long before any being appeared on the surface of the earth capable of turning their power to account, they are sources of power no longer. And here we might halt for a moment to remark on that tendency so prevalent in the world, to regard everything as made for human use Those who entertain this notion hold. I think, an overweening opinion of their own importance in the system of nature. Flowers bloomed before men saw them, and the quantity of power wasted before men could utilise it is all but infinite compared with what now remains to be applied. The healthy attitude of mind with reference to this subject is that of the poet, who, when asked whence came the rhododendron, replied—
I never thought to ask, I never knew.
But in my simple ignorance supposed
The self-same power that brought me there brought you.”
A few exceptions to this general state of union of the particles of the earth’s crust—all-important to us, but trivial in comparison to the total store of which they are but the residue—still remain. They constitute our main sources of motive power. By far the most important of these exceptions are our beds of coal, composed chiefly of carbon, which has not yet closed in chemical union with oxygen. Distance still intervenes between the atoms of carbon and those of oxygen, across which the atoms may be impelled by their mutual attractions; and we can do nothing more than utilise the motion produced by this attraction. Once the carbon and the oxygen have closed together, so as to form carbonic acid, their mutual attractions are satisfied; and while they continue in this condition, as dynamic agents they are dead. Our woods and forests are sources of mechanical energy, because they also have the power of uniting with the atmospheric oxygen, and the molecular motion produced in the act of union may be turned to mechanical account. And let it be remembered that the source of motive power here referred to is also the source of muscular power. A horse can perform work, and so can a man; but this work is at bottom the molecular work of the elements of the food and the oxygen of the air. We inhale this vital gas. We bring it into sufficiently close proximity with the carbon and the hydrogen of the food. They unite in obedience to their mutual attractions, and their motion towards each other, properly turned to account by the wonderful mechanism of the body, becomes muscular motion.
One fundamental thought pervades all these statements: there is one tap-root from which they all spring. This taproot is the ancient maxim that out of nothing nothing comes; that neither in the organic world nor in the inorganic is power produced without the expenditure of other power; that neither in the plant nor in the animal is there a creation of force or motion. Trees grow, and so do men and horses; and here we have new power incessantly introduced upon the earth. But its source, as I have already stated, is the sun. For he it is who separates the carbon from the oxygen of the carbonic acid, and thus enables them to recombine. Whether they recombine in the furnace of the steam-engine or in the animal body, the origin of the power they produce is the same. In this sense we are all “souls of fire and children of the sun.” But, as remarked by Helmholtz, we must be content to share our celestial pedigree with the meanest living thing. The frog, and the toad, and those terrible things, the monkey and the gorilla, draw their power from the same source as man.
Some estimable persons here present very possibly shrink from accepting these statements; they may be frightened by their apparent tendency towards what is called materialism—a word which to many minds expresses something very dreadful. But it ought to be known and avowed that the physical philosopher, as such, must be a pure materialist. His inquiries deal with matter and force, and with them alone. The action which he has to investigate is necessary action, not spontaneous action—the transformations, and not the creation, of matter and force. And whatever be the forms which matter and force may assume, whether in the organic world or the inorganic, whether in the coal beds and forests of the earth or in the brains and muscles of men, the physical philosopher will make good his claim to investigate them. It is perfectly vain to attempt to stop investigation as to the actual and possible combinations of matter and force. Depend upon it, if a chemist, by bringing the proper materials together, could produce a baby he would do it. And why not? There is no command forbidding him to do it—his inquiries in this direction are limited solely by his own capacity and the inexorable laws of matter and force. At the present moment there are, no doubt, persons experimenting on the possibilities of producing what we call life out of inorganic materials. Let them pursue their studies in peace; it is [p.259] only by such trials that they will learn the limits of their powers.
But while I thus make the largest claim for freedom of investigation—while 1 as a man of science feel a natural pride in scientific achievement, while I regard science as the most powerful instrument of intellectual culture, as well as the most powerful ministrant to the material wants of men, if you ask me whether science has solved, or is likely to solve, the problem of this universe, I must shake my head in doubt. We have been talking of matter and force; but whence came matter, and whence came force? You remember the first Napoleon’s question, when the savans who accompanied him to Egypt discussed in his presence the problem of the universe, and solved it to their apparent satisfaction. He looked aloft to the starry heavens, and said—“It is all very well, gentlemen, but who made all these?” That question still remains unanswered, and science makes no attempt to answer it. As far as I can see, there is no quality in the human intellect which is lit to be applied to the solution of the problem. It entirely transcends us.
The mind of man may be compared to a musical instrument with a certain range of notes, beyond which in both directions we have an infinitude of silence. The phenomena of matter and force lie within our intellectual range, and as far as they reach we will at all hazards push our enquiries. But behind, and above, and around all, the real mystery of this universe remains unsolved; and here the true philosopher will bow his head in humility, and admit that all he can do in this direction is no more than what is within the compass of an ordinary child. Fashion this mystery as you will, with that I have nothing to do. But be careful that your conception of the Builder of this universe is not an unworthy conception. Invest that conception with your grandest, and highest, and holiest thought, but be careful of pretending to know more about it than is given to man to know. Be careful, above all things, of professing to see in the phenomena of the material world the evidences of Divine pleasure or displeasure. Doubt this all ye who would deduce from the fall of the Tower of Siloam, the sin and wickedness of those who were crushed by the fall! Doubt this all ye who pretend to see in cholera, cattle plague, and bad harvests, the evidence of Divine anger! Doubt this—but it requires some courage to say these things in Scotland—all ye who assert that the depreciation of railway scrip is a consequence of railway travelling on Sundays. You know nothing about it. To such I say in substance, what was said by one of the mightiest Scotchmen living or dead—Thomas Carlyle—to the followers of Dr. Pusey—
He formed all souls, all systems, planets, particles;
The plan he formed his worlds and Æons by,
Was—Heavens!—was thy small nine-and-thirty articles!”
- Science Quotes by John Tyndall.
- 2 Aug - short biography, births, deaths and events on date of Tyndall's birth.
- John Tyndall - context of quote “Fatal…to blink facts” - Medium image (500 x 250 px)
- John Tyndall - context of quote “Fatal…to blink facts” - Large image (800 x 400 px)
- John Tyndall - context of quote “The First Experiment a Child Makes” - Medium image (500 x 250 px)
- John Tyndall - context of quote “The First Experiment a Child Makes” - Large image (800 x 400 px)
- A Vision of Modern Science: John Tyndall and the Role of the Scientist in Victorian Culture, by Ursula DeYoung. - book suggestion.