 (source)
(source)
|
Dmitry Ivanovich Mendeleev
(8 Feb 1834 - 2 Feb 1907)
Russian chemist who developed the periodic classification of the elements.
|
Science Quotes by Dmitry Ivanovich Mendeleev (9 quotes)
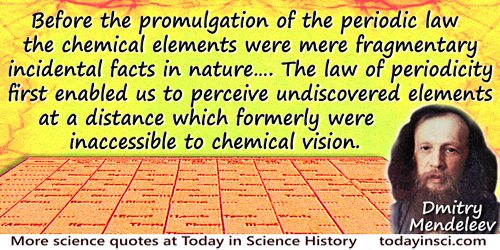
Before the promulgation of the periodic law the chemical elements were mere fragmentary incidental facts in nature; there was no special reason to expect the discovery of new elements, and the new ones which were discovered from time to time appeared to be possessed of quite novel properties. The law of periodicity first enabled us to perceive undiscovered elements at a distance which formerly were inaccessible to chemical vision, and long ere they were discovered new elements appeared before our eyes possessed of a number of well-defined properties.
— Dmitry Ivanovich Mendeleev
In Faraday Lecture, delivered before the Fellows of the Chemical Society in the Theatre of the Royal Institution (4 Jun 1889), printed in Professor Mendeléeff, 'The Periodic Law of the Chemical Elements', Transactions of the Chemical Society (1889), 55, 648.
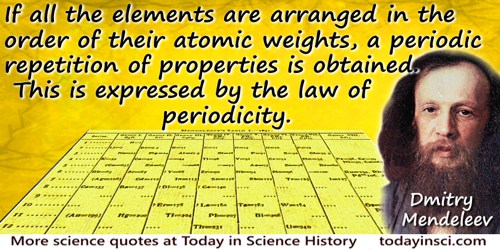
If all the elements are arranged in the order of their atomic weights, a periodic repetition of properties is obtained. This is expressed by the law of periodicity.
— Dmitry Ivanovich Mendeleev
Principles of Chemistry (1905), Vol. 2, 17.
In that pure enjoyment experienced on approaching to the ideal, in that eagerness to draw aside the veil from the hidden truth, and even in that discord which exists between the various workers, we ought to see the surest pledges of further scientific success. Science thus advances, discovering new truths, and at the same time obtaining practical results.
— Dmitry Ivanovich Mendeleev
In The Principles of Chemistry (1891), Vol. 1, preface, footnote, ix, as translated from the Russian 5th edition by George Kamensky, edited by A. J. Greenaway.
Knowing how contented, free and joyful is life in the realms of science, one fervently wishes that many would enter their portals.
— Dmitry Ivanovich Mendeleev
In The Principles of Chemistry (1891), Vol. 1, preface, footnote, ix, as translated from the Russian 5th edition by George Kamensky, edited by A. J. Greenaway.
Science, which deals with the infinite, is itself without bounds.
— Dmitry Ivanovich Mendeleev
In The Principles of Chemistry (1891), Vol. 1, preface, footnote, x, as translated from the Russian 5th edition by George Kamensky, edited by A. J. Greenaway.
The edifice of science not only requires material, but also a plan. Without the material, the plan alone is but a castle in the air—a mere possibility; whilst the material without a plan is but useless matter.
— Dmitry Ivanovich Mendeleev
In The Principles of Chemistry (1891), Vol. 1, preface, footnote, ix, as translated from the Russian 5th edition by George Kamensky, edited by A. J. Greenaway.
There must be some bond of union between mass and the chemical elements; and as the mass of a substance is ultimately expressed (although not absolutely, but only relatively) in the atom, a functional dependence should exist and be discoverable between the individual properties of the elements and their atomic weights. But nothing, from mushrooms to a scientific dependence can be discovered without looking and trying. So I began to look about and write down the elements with their atomic weights and typical properties, analogous elements and like atomic weights on separate cards, and soon this convinced me that the properties of the elements are in periodic dependence upon their atomic weights; and although I had my doubts about some obscure points, yet I have never doubted the universality of this law, because it could not possibly be the result of chance.
— Dmitry Ivanovich Mendeleev
Principles of Chemistry (1905), Vol. 2, 18.
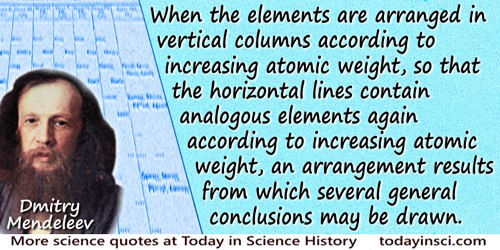
When the elements are arranged in vertical columns according to increasing atomic weight, so that the horizontal lines contain analogous elements again according to increasing atomic weight, an arrangement results from which several general conclusions may be drawn.
— Dmitry Ivanovich Mendeleev
'The Relations of the Properties to the Atomic Weights of the Elements', Zeitschrift fur Chemie, 1869.
Why do they [Americans] quarrel, why do they hate Negroes, Indians, even Germans, why do they not have science and poetry commensurate with themselves, why are there so many frauds and so much nonsense? I cannot soon give a solution to these questions ... It was clear that in the United States there was a development not of the best, but of the middle and worst sides of European civilization; the notorious general voting, the tendency to politics... all the same as in Europe. A new dawn is not to be seen on this side of the ocean.
— Dmitry Ivanovich Mendeleev
The Oil Industry in the North American State of Pennsylvania and in the Caucasus (1877). Translated by H. M. Leicester, from the original in Russian, in 'Mendeleev's Visit to America', Journal of Chemical Education (1957), 34, 333.
See also:
- 8 Feb - short biography, births, deaths and events on date of Mendeleev's birth.
- A Footnote on Science - by Dmitry Mendeleev in Principles of Chemistry (1891).
- Dmitry Ivanovich Mendeleyev: His Life and His Work, by O. N. Pisarzhevsky. - book suggestion.
- Booklist for Dmitry Mendeleyev.







 In science it often happens that scientists say, 'You know that's a really good argument; my position is mistaken,' and then they would actually change their minds and you never hear that old view from them again. They really do it. It doesn't happen as often as it should, because scientists are human and change is sometimes painful. But it happens every day. I cannot recall the last time something like that happened in politics or religion.
(1987) --
In science it often happens that scientists say, 'You know that's a really good argument; my position is mistaken,' and then they would actually change their minds and you never hear that old view from them again. They really do it. It doesn't happen as often as it should, because scientists are human and change is sometimes painful. But it happens every day. I cannot recall the last time something like that happened in politics or religion.
(1987) -- 


